Cell & Gene Therapy Insights is an online, open-access, peer-reviewed journal dedicated to the interdisciplinary exploration and advancement of cell and gene therapy. With a translational focus, we connect innovative research to practical clinical applications, providing valuable insights into one of biotechnology’s fastest-evolving fields.
We address the key challenges and latest developments across advanced therapies, publishing original research articles, expert reviews, commentary, clinical trial reports, and more. Visit the Cell & Gene Therapy Insights Journal page for our complete collection.
Our popular webinar series provides expert-led discussions on important developments and methodologies in the field, designed to support ongoing professional growth.
Explore our specialised channels, including detailed coverage of critical areas like the cell and gene supply chain, for targeted insights into manufacturing, logistics, and regulatory frameworks essential to therapy commercialisation.
Learn more about our journal’s mission and publishing criteria by visiting our aims and scope.
Sign-up for free to gain unlimited access to our extensive library of articles, webinars, podcasts, news, and interviews, and stay at the forefront of cell and gene therapy innovation.
If you’re interested in working with us, from sponsoring articles to webinars and more, view the media kit to find out more.
December 2025
Upcoming webinars

Developing streamlined, flexible cell therapy process workflows: from upstream to formulation and fill-finish operations

Genomic integrity and safety assessment strategies for CRISPR-edited cell therapies
Defining effective antitumor T cell responses to guide next-generation adoptive cell therapies for solid tumors
Steady and stable transfection from lab to launch: enabling seamless viral vector scale-up
Advancing non-viral CAR-NK development: LNP engineering insights and cell therapy regulatory trends
Frozen by design: engineering and automating cryogenic storage to avoid risk and scale-up surprises
Latest Articles
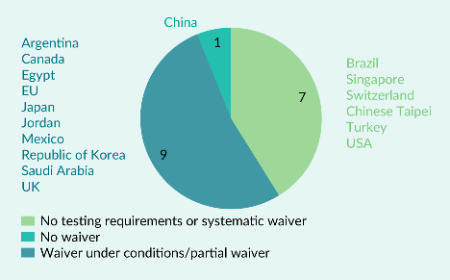
An analysis of advanced therapy medicinal products regulatory landscape in ICH member countries: opportunities and challenges

The cryo-sensitivity of NK cells: overcoming post-thaw decline

Integration site analysis in engineered T cell therapies: what we measure, what we miss, and what regulators expect

Systemization is key to fulfilling T cell therapy’s promise in cancer
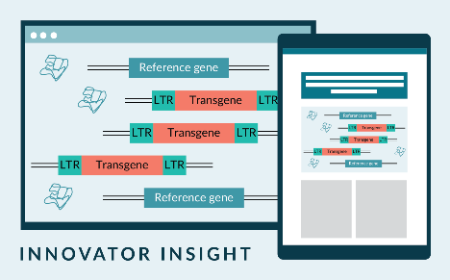
Streamlined dPCR workflow for lentiviral characterization and QC of cell and gene therapies

Role of supportive care in the future of cell therapies
In vivo CAR therapy: quo vadis?

Advocacy at warp speed: delivering the first gene replacement therapy for SLC6A1