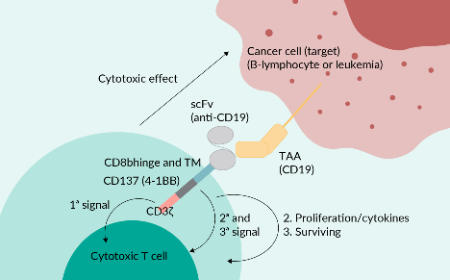
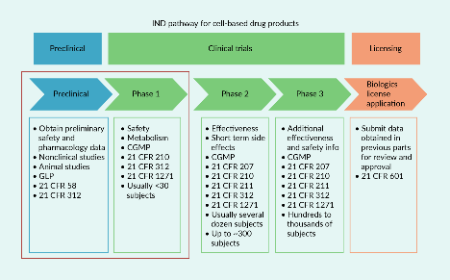
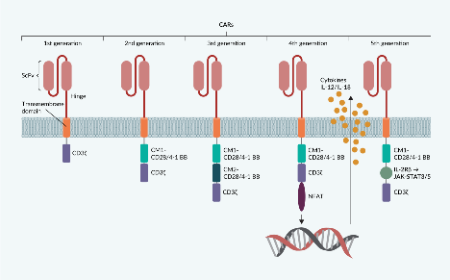
Cell & Gene Therapy Insights is an online, open-access, peer-reviewed journal dedicated to the interdisciplinary exploration and advancement of cell and gene therapy. With a translational focus, we connect innovative research to practical clinical applications, providing valuable insights into one of biotechnology’s fastest-evolving fields.
We address the key challenges and latest developments across advanced therapies, publishing original research articles, expert reviews, commentary, clinical trial reports, and more. Visit the Cell & Gene Therapy Insights Journal page for our complete collection.
Our popular webinar series provides expert-led discussions on important developments and methodologies in the field, designed to support ongoing professional growth.
Explore our specialised channels, including detailed coverage of critical areas like the cell and gene supply chain, for targeted insights into manufacturing, logistics, and regulatory frameworks essential to therapy commercialisation.
Learn more about our journal’s mission and publishing criteria by visiting our aims and scope.
Sign-up for free to gain unlimited access to our extensive library of articles, webinars, podcasts, news, and interviews, and stay at the forefront of cell and gene therapy innovation.
If you’re interested in working with us, from sponsoring articles to webinars and more, view the media kit to find out more.
October 2025
Upcoming webinars
Advancing RNA production: real-time quality, predictive control, and the future of intelligent biomanufacturing
Accelerating digitization in cell & gene therapy manufacturing: real-world insights & practical strategies
Enzymatic versus plasmid DNA for rAAV production: early development insights that lead to manufacturing success

Quality agreements, tech transfer, and risk management in cell and gene therapy
iPSC biomanufacturing: advances in scalable technologies with fixed bed bioreactor systems
Latest Articles
Development and scale-up of a transient transfection rAAV process to a next-generation, single-use bioreactor system
De-risking clinical trials and enabling innovation: key strategies for empowering emerging biotechs

Advanced Therapies Week 2026

Bringing gene therapy to the brain: Cardiff’s role in the landmark Huntington’s trial
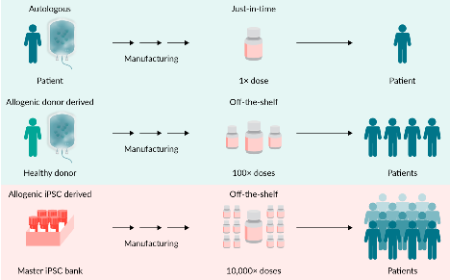
Genetic integrity testing of genome-edited pluripotent stem cell lines used for cell therapy applications

Cryo-processed leukapheresis using an automated closed system: a high-quality starting material for autologous and allogeneic CAR-T cell therapy manufacturing

Building scalable, science-forward gene delivery platforms
Beyond the liver: reimagining delivery systems for the future of gene and RNA medicines