Cell & Gene Therapy Insights is an online, open-access, peer-reviewed journal dedicated to the interdisciplinary exploration and advancement of cell and gene therapy. With a translational focus, we connect innovative research to practical clinical applications, providing valuable insights into one of biotechnology’s fastest-evolving fields.
We address the key challenges and latest developments across advanced therapies, publishing original research articles, expert reviews, commentary, clinical trial reports, and more. Visit the Cell & Gene Therapy Insights Journal page for our complete collection.
Our popular webinar series provides expert-led discussions on important developments and methodologies in the field, designed to support ongoing professional growth.
Explore our specialised channels, including detailed coverage of critical areas like the cell and gene supply chain, for targeted insights into manufacturing, logistics, and regulatory frameworks essential to therapy commercialisation.
Learn more about our journal’s mission and publishing criteria by visiting our aims and scope.
Sign-up for free to gain unlimited access to our extensive library of articles, webinars, podcasts, news, and interviews, and stay at the forefront of cell and gene therapy innovation.
If you’re interested in working with us, from sponsoring articles to webinars and more, view the media kit to find out more.
May 2025
Upcoming webinars
Learn how to design a simplified, cost-effective LVV manufacturing workflow
Optimizing iPSC-derived cell therapy development
Future-proofing gene therapy: scalable AAV solutions and non-viral innovations

Strategic human raw material selection for cell therapy manufacturing

Cell therapy clinical translation: ensuring speed to clinic while mitigating risk
Exploring scalable, data-driven AAV manufacturing to achieve high yield and quality across serotypes
Latest Articles
Exploring asprosin: how AAV vectors are advancing hormone research

Rewriting the code: examining the promise and responsibility of gene editing

Targeting T cells in vivo: advances in non-viral gene delivery and clinical applications

Understanding and mitigating risks associated with cryopreservation practices in cell therapy
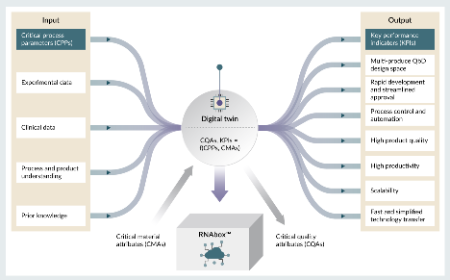
Overcoming mRNA medicine supply hurdles: distributed, continuous and multi-product mRNA manufacturing in a box at high quality and low cost

A practical guide to fit-for-purpose analytical development in an emerging cell therapy landscape

Exploring the UK’s evolving role in commercializing cell and gene therapies

Key considerations for scalable retroviral vector production—with a focus on lentivirus