Celyad Oncology’s case study: validation approach for accelerated mycoplasma testing
Cell & Gene Therapy Insights 2021; 6(10), 1755–1764
10.18609/cgti.2020.193
Underneath the development and launch of cell therapy products lies a highly complex supply chain, and clinical manufacturers of cell therapies must ensure quality control from the starting material through to product delivery to the patient. Before a product can make it to the clinic, it must meet predefined criteria that confirm safety, quality and efficacy. Quality management and release criteria, including microbiological safety, form a crucial part of this process. Within this, mycoplasma testing can pose a challenge, as the classical analytical methods documented within the US and European guidances involve challenging manual techniques with significant turnaround times. Alternative testing approaches are allowed, but they must be validated and studied in comparison to established mycoplasma testing methods. In this case study, the benefits and performance of the Roche MycoTOOL qPCR test are discussed.
Accelerated mycoplasma testing: the need
As a company, Celyad Oncology is working to discover and develop innovative allogeneic and autologous CAR T products to fight cancer, with a number of pioneering CAR T approaches under development. Of course, before a cell therapy can be brought into the clinic it must meet predefined acceptance criteria that confirm safety, quality, and efficacy. Microbiological testing, and more specifically mycoplasma testing, is a crucial part of the characterization and release process.
Both the European Pharmacopeia 2.6.7 and the US Pharmacopeia 63 recommend two different methods; the culture method, and the indicator cell culture method.
The culture method is based on an extension in broth culture, and a detection in agar. While the limit of detection (LOD) is sensitive (0.1 CFU/mL), the turnaround time for this test is about 28 days. Only culturable mycoplasmas can be detected, and often a very large volume is required. The test is manual and not automated.
The other option is the indicator cell culture method, which involves an extension in cell culture, and afterwards characteristic fluorescent staining of the DNA. Using this method mycoplasma can be detected by its characteristic pattern, and non-cultivable mycoplasma can also be detected. The limitation is again a long turnaround time, in this case of about 10 days. The LOD is only 100 CFU/mL, and this approach poses a challenging manual method.
However, both the Pharmacopeia and USP leave the door open for alternative testing. The European Pharmacopeia is very precise – a validated nucleic acid amplification technique (NAT) test is recommended. It must be validated, and a comparability study against the classical methods must be done.
The benefit of a NAT over classical approaches is that it is significantly faster, no cultivation is needed, and the LOD of the technique is at least 10 CFU/mL. There are currently several commercially available alternative approaches for mycoplasma testing available.
Choosing an alternative mycoplasma test: the MycoTOOL kit
Celyad Oncology made the decision to move forward with the MycoTOOL qPCR test from Roche, due to several advantages it offered. The method is fully validated by Roche, and validation reports can be accessed under an NDA. The set-up and validation is performed on a LightCycler® system that is already in use at Celyad Oncology. The method is fit for automation from the DNA extraction to the PCR steps, making it sustainable in the long term. Finally, it is applicable for both cellular and non-cellular matrices, so can also be used for raw material release.
However, these advantages are contrasted against some limitations. Currently the test is only validated for up to 5 million cells per mL, which means an alternative sampling strategy may be required depending on product volume and concentration. The automated extraction method is validated on the Roche MagNA Pure 96 system. Both the MagNA Pure 24 system and the QC Preparation Kit are functionally tested, but not validated. If another system is used, it would first need to be validated.
The workflow of the MycoTOOL is shown in Figure 1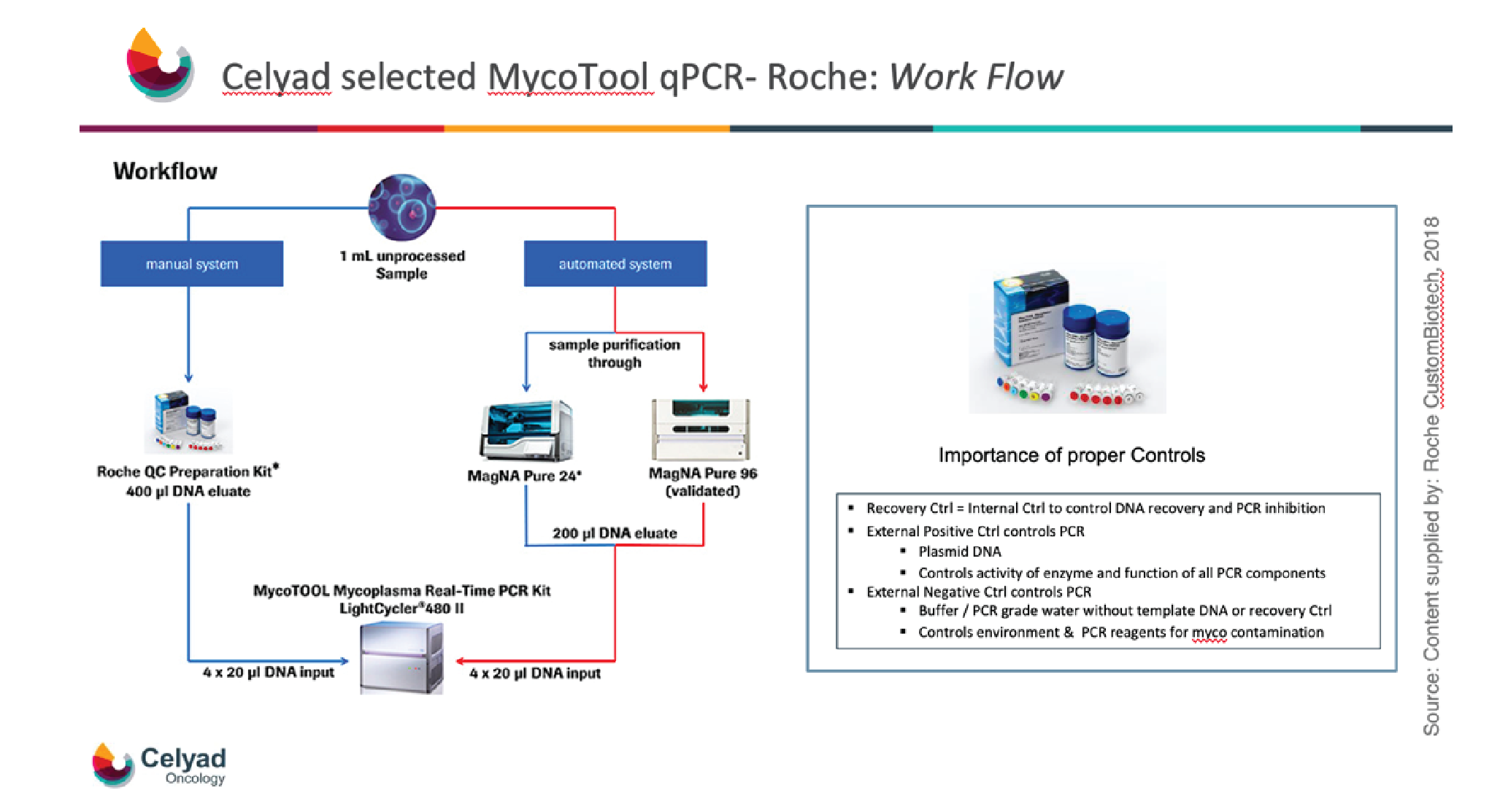
The use of proper controls are critical to the workflow, and all required controls are provided within the kit. The recovery control is used to control DNA extraction, recovery, and PCR formation. The external positive control again controls PCR, allowing control of the activity of the enzyme and also the function of all the PCR components. The external negative control is a buffer or PCR grade water without any templates, to control for the environment and equipment, and also for PCR reagents, to ensure that all components are mycoplasma free.
MycoTOOL validation
When choosing an alternative method for mycoplasma testing, the first step is validation. The MycoTOOL from Roche, as discussed above, is fully validated according to the requirements of pharmacopoeia 2.6.7. and also ICH Q2 R1, in terms of robustness, precision, specificity, sensitivity, cross-contamination, and comparability versus classical methods.
Additionally, parts of the validation from the manufacturer can replace the validation by the user, as per Ph Eur 2.6.7:
“When the analytical procedure is fully or partly implemented using commercial kits, aspects of validation already supported by the manufacturer, supporting documentation being available, can be omitted by the user. Nevertheless, this one must demonstrate the performances of the kit related to the intended use.”
From the guidance, it is clear that the user must demonstrate the performance of the kit related to the intended use. This involves assessing the matrix inhibitory or interference properties at the length of detection, i.e., 10 CFU/mL.
Figure 2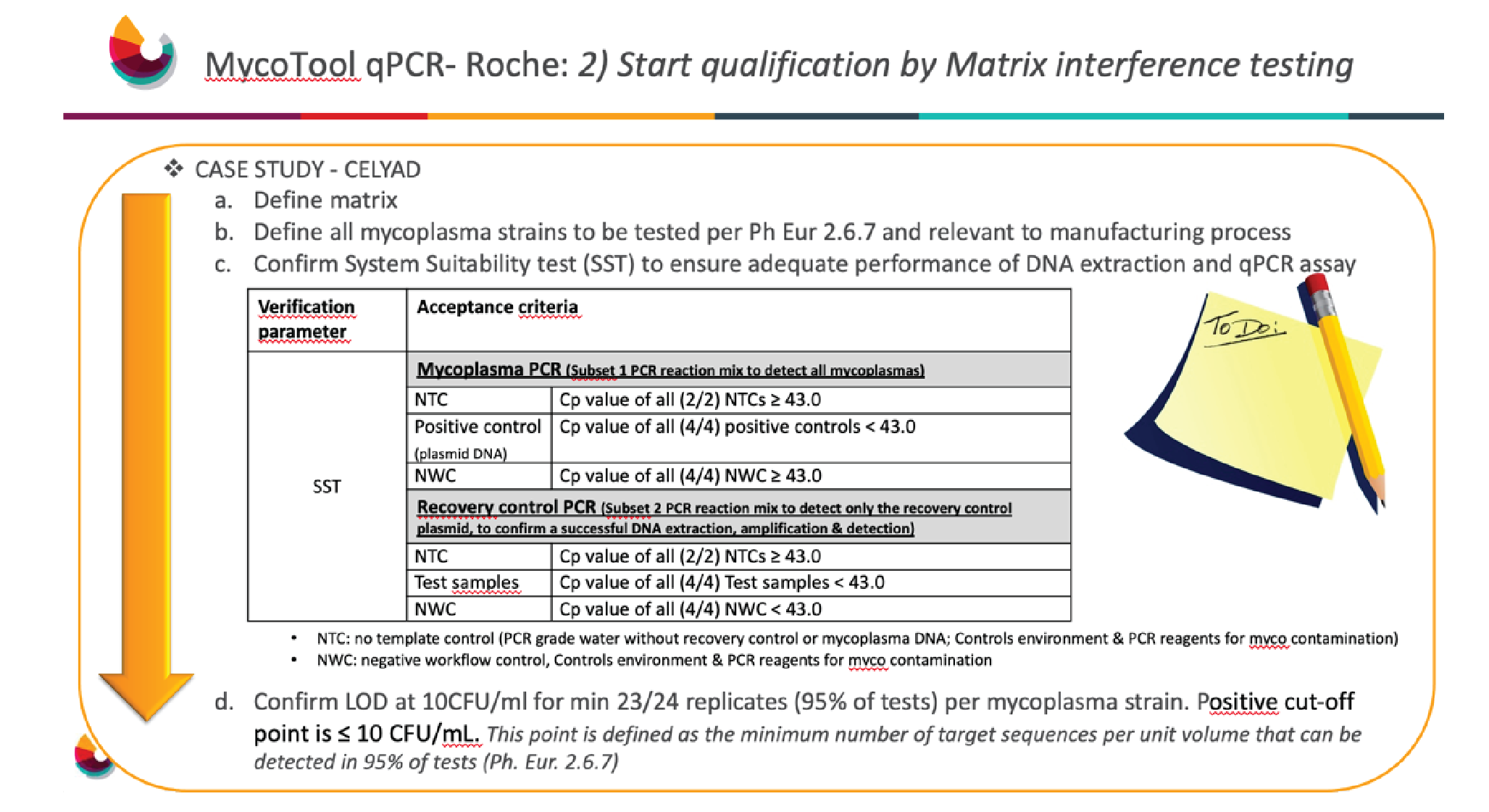
A system suitability test must be performed to ensure adequate performance of the DNA extraction and qPCR asset. With MycoTOOL there are two different subsets – one focuses on mycoplasma, and the other focuses on recovery control. The recovery control is added to all samples except for the non-template control and positive control. This provides assurance of a successful DNA extraction and mycoplasma detection, so that in case of a negative result the user can be confident this is not a false negative.
The positive control, a plasmid DNA, is to ensure any mycoplasma present is being detected. There is also the non-template control, which as discussed above, is needed to ensure that the environment, equipment and PCR reagents are indeed free from mycoplasma.
Once testing is completed, the next step is to confirm a LOD of 10 CFU/mL for a minimum of 23 out of 24 replicates, i.e., a detection capability of 95% per mycoplasma strain. In Celyad Oncology’s case, a target of 24 out of 24 was chosen in order to achieve 100% detection capability.
Celyad Oncology Oncology produces some cryopreserved products at concentrations higher than 5 million cells per ml. In order to increase sensitivity, a fresh sample was used that consisted of cells and cell culture supernatants at harvest. Cells were included in the testing to increase sensitivity, as mycoplasma may also be present within them.
Next, the mycoplasma strains that are recommended per the pharmacopoeia, but were also relevant to the manufacturing process, had to be defined. As Celyad Oncology does not use avian raw material or material from a plant or insect source, Mycoplasma gallisepticum, Mycoplasma synoviae, and Mycoplasma citri were excluded, and the other six strains listed in the Ph Eur 2.6.7 were tested (Figure 3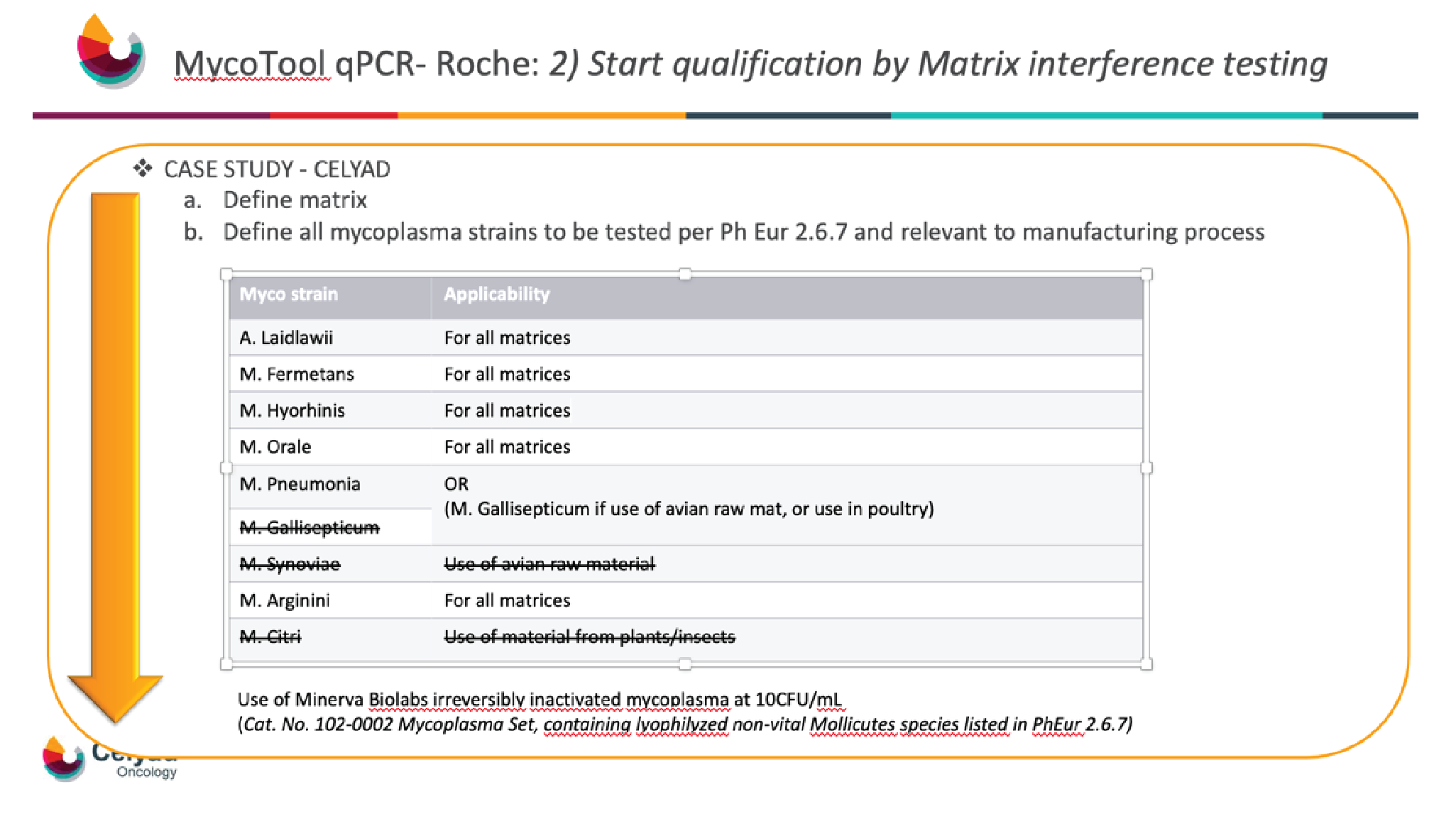
In order to be able to do the whole qualification at Celyad Oncology in the GMP QC laboratory of Celyad Oncology, and not contaminate the laboratory with mycoplasma, inactivated mycoplasma reconstituted at 10 CFU/mL was chosen.
With the mycoplasma and matrix selected and the system suitability test completed, the next significant task was the LOD. The 6 spiked mycoplasmas were detected at 10CFU/mL, and detection was achieved for 4 out of 4 replicates, resulting in a pass (Figure 4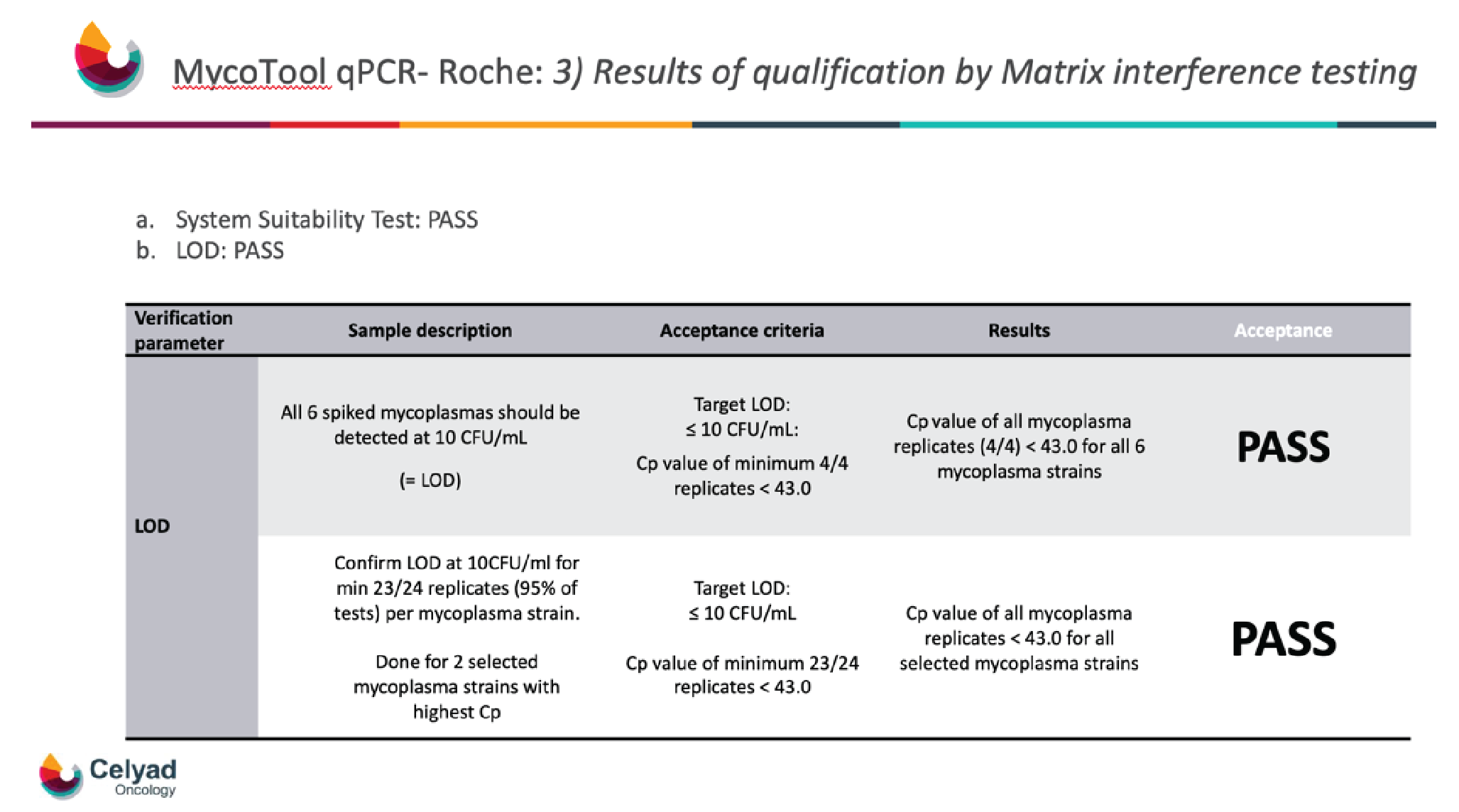
Conclusion
Fast-track mycoplasma tests offers a range of advantages and can be useful for both autologous and allogeneic cell therapies. Their rapid outcomes allow for both quick in-process control and product release, and they can assist manufacturers in overcoming technical limitations. They are also suitable for in-house and automated testing.
When considering whether to adopt an alternative mycoplasma testing approach, a risk-based strategy is the best option. It is also important to fully understand what regulatory bodies expect – for example, the guidance discussed above lists a number of mycoplasma strains, but it is only necessary to test those which are relevant to your manufacturing process. It is crucial to work in partnership with regulatory authorities, in order to ensure a reliable and sensitive alternative method which will enable the development of high-quality products.
Q & A
Q What are the chief considerations for Celyad Oncology’s autologous and allogeneic cell therapy product candidates in terms of biological testing?
SS: In my personal opinion, it is critical to do a risk assessment for ICHQ9.
It is true that for all microbiologic testing, the general rule remains that you have to follow the compendial monographs. But for cell therapy products, this is not always feasible. The good news is that the regulatory landscape is moving forward, and it is going in the right direction and opening new doors for us around rapid alternative testing. This is especially the case for short lived products and also for cell therapy products that cannot be assessed by compendial methods due to technical limitations such as volume, size, or concentration.
It is important that these fast alternative methods are fully validated, that proper controls are used, and that the methods and validation are fit for purpose – and you also have to look into the matrix interference properties. We fully validate in terms of method sensitivity, specificity, and also reproducibility.
Q Can you go deeper into the specific drivers behind Celyad Oncology’s search for an alternative mycoplasma testing solution?
SS: There are three main drivers. Firstly, having a very sensitive test that can be easily validated and also easily transferred. Secondly, having a test that allows rapid in-process detection, but also rapid release of our autologous products. Finally, having also a method that is sustainable in the long term, that can be internalized, and is also fit for automation.
Q Which sample types are most suitable for the test?
SS: This is a very individual answer, because you have to know your product. For instance, if you have a cell therapeutic product, personally I would recommend both cell culture supernatants and also the cells themselves, because the mycoplasma can actually also be present within the cells.
On the other hand, if you have a cryopreserved method you want to have a detection that is as sensitive as possible, so here we recommend to do the testing on a fresh sample.
Q How long can your sample for testing be stored?
SS: This is again very individual, and you have to test that for yourself. I would highly recommend when you do this kind of validation that you also include stability shelf life testing.
Having now successfully qualified an alternative mycoplasma testing approach, what advice you would have for other companies that are interested in implementing a rapid microbial testing approach for their therapy?
SS: You have to follow a risk-based approach, I cannot emphasize that enough. Ensure that your method as well as your validation is fit-for-purpose. You have to use the proper controls – don’t just read the guidelines; ensure they are relevant for your manufacturing process. Don’t be afraid to start this kind of validation, but use it wisely.
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Regulatory Disclaimer: For use in quality control/manufacturing process only.
LightCycler® 480 Instrument: For Life Science Research Use Only. Not for use in diagnostic procedures.
Trademarks: MycoTOOL, MAGNA PURE, and LIGHTCYCLER are trademarks of Roche. All other product names and trademarks are the property of their respective owners.
Disclosure and potential conflicts of interest: The author declares that they have no conflicts of interest.
Funding declaration: Sarah Snykers has been paid a fee by Roche Diagnostics for her participation in this interview.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2021 Roche Diagnostics Corporation. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: This article is a transcript of a previously published webinar, which can be found here.
Webinar published: Jan 7 2021; Publication date: Feb 16 2021.



