Breaking viral vector bioanalysis barriers with centrifugal force
Cell & Gene Therapy Insights 2021; 7(2), 223–232
10.18609/cgti.2021.005
Advances in viral vector gene delivery systems, particularly adeno-associated virus (AAV) and lentivirus (LV), have accelerated the development of new cell and gene therapies. Regulatory programs for accelerated review have added to the demand for the manufacture of viral vectors, exceeding capacity and creating backlogs. Improvements in analysis speed, accuracy, precision, and dynamic range are potential targets for accelerating production timelines. Plate-based enzyme-linked immunosorbent assays (ELISAs), commonly used in analysis of viral vector titer, purity, and potency, have laborious and time-consuming manual processing drawbacks as well as long processing times and poor precision. A novel automated microfluidic, compact disc (CD)-based immunoassay format that uses centrifugal force to precisely control the flow of sample and reagents has been notably effective in accelerating bioanalysis of antibody-based therapeutics with high-precision results. One-hour assay run times and wide dynamic ranges accelerate workflows, and 10 µL sample requirements minimizes consumption of limited production material. These dramatic immunoassay improvements are expected to alleviate the analytical delays in viral vector manufacturing.
Cell and gene therapies have brought the promise of effective, even curative, therapies for acute or chronic genetic diseases where no treatment or only long-term symptomatic treatment exists. Over the past decade, advances in viral vector delivery systems, along with programs facilitating development of gene therapies and immunotherapies, have fanned the flames of an already active field [1]Lapteva L, Purohit-Sheth T, Serabian M, Puri RK. Clinical Development of Gene Therapies: The First Three Decades and Counting. Mol. Ther. Meth. Clin. Dev. 2020; 19: 387–97. , [2]Ma CC, Wang ZL, Xu T, He ZY, Wei YQ. The approved gene therapy drugs worldwide: from 1998 to 2019. Biotechnol. Adv. 2020; 40: 10750 . Programs being actively promoted by regulatory bodies such as the Medicines and Healthcare Products Regulatory Agency (MHRA) and the United States Food and Drug Administration (FDA) include expedited development and review, fast-track designation, accelerated approval, and breakthrough therapy designation, all focused on accelerating therapies treating unmet medical needs. This further availability of shortened development timelines and financial benefits has attracted pharma and biotech companies to invest in the development of genetic therapies with rigorously scheduled development plans.
These incentive programs have also had a noticeable effect on the expansion of the gene therapy pipeline – in just the last 2 years, the number of cell and gene therapies in clinical development has grown from 289 to 362, or a 25% increase [3]. In addition, the number of gene therapy clinical trials is expected to continue its meteoric rise from 775 in 2019 to >4,000 ongoing or completed in 2020 [4]Maldonado R, Jalil S, Wartiovaara K. Curative gene therapies for rare diseases. J. Commun. Genet. 2020: 1–10. , projected to be nearly 11,000 by 2026 [5]Rininger J, Fennell A, Schoukroun-Barnes L, Peterson C, Speidel J. Capacity Analysis for Viral Vector Manufacturing Is There Enough? BioProcess Int. 2019; 17(11). Rininger J, Fennell A, Schoukroun-Barnes L, Peterson C, Speidel J. Capacity Analysis for Viral Vector Manufacturing Is There Enough? BioProcess Int. 2019; 17(11). Rininger J, Fennell A, Schoukroun-Barnes L, Peterson C, Speidel J. Capacity Analysis for Viral Vector Manufacturing Is There Enough? BioProcess Int. 2019; 17(11). , [6]Quinn C, Young C, Thomas J, Trusheim M. Estimating the Clinical Pipeline of Cell and Gene Therapies and Their Potential Economic Impact on the US Healthcare System. Value in Health 2019; 22(6): 621– . Lentivirus (LV) and adeno-associated virus (AAV) vectors are the most successful delivery systems for cell and gene therapies, and recent regulatory approvals utilizing these vectors for generation of chimeric antigen receptor (CAR) T cell cancer immunotherapies include: Tecartus™ (brexucabtagene autoleucel, Kite, a Gilead company) for treatment of mantle cell lymphoma, Yescarta® (axicabtagene ciloleucel, Gilead Sciences, Inc.) for treatment of relapsed or refractory large B-cell lymphoma, and Kymriah® (tisagenlecleucel, Novartis) for treatment of B-cell acute lymphoblastic leukemia.
AAV vectors have risen in popularity for systemic delivery of genetic therapies primarily because of their small size, tissue tropism, and low immunogenicity. Recent AAV approvals include Luxturna® (voretigene neparvovec-rzyl, Spark Therapeutics) for RPE65 mutation-associated retinal dystrophy, and Zolgensma® (onasemnogene abeparvovec-xioi, AveXis, Inc) for the treatment of pediatric patients with spinal muscular atrophy (SMA).
Breaking the manufacturing bottlenecks
The popularity of AAV and LV vectors, along with the recent swell in the development pipeline for cell and gene therapies, has outstripped manufacturing capacity and created backlogs in the bioreactor production, along with compressed development and production timeline pressures [5]Rininger J, Fennell A, Schoukroun-Barnes L, Peterson C, Speidel J. Capacity Analysis for Viral Vector Manufacturing Is There Enough? BioProcess Int. 2019; 17(11). Rininger J, Fennell A, Schoukroun-Barnes L, Peterson C, Speidel J. Capacity Analysis for Viral Vector Manufacturing Is There Enough? BioProcess Int. 2019; 17(11). Rininger J, Fennell A, Schoukroun-Barnes L, Peterson C, Speidel J. Capacity Analysis for Viral Vector Manufacturing Is There Enough? BioProcess Int. 2019; 17(11). , [7]Gene Therapies Push Viral Vector Production – Bioprocess Development Forum: http://www.processdevelopmentforum.com/articles/gene-therapies-push-viral-vector-production/Gene Therapies Push Viral Vector Production – Bioprocess Development Forum: http://www.processdevelopmentforum.com/articles/gene-therapies-push-viral-vector-production/.
Analytical characterization of viral vector identity, potency, purity, safety, and stability during manufacturing can contribute significantly to the overall timeline for vector therapeutic production [8]Transfiguracion J, Tran MY, Lanthier S et al. Rapid In-Process Monitoring of Lentiviral Vector Particles by High-Performance Liquid Chromatography. Mol. Ther. Meth. Clin. Dev. 2020; 18: 803–10. Transfiguracion J, Tran MY, Lanthier S et al. Rapid In-Process Monitoring of Lentiviral Vector Particles by High-Performance Liquid Chromatography. Mol. Ther. Meth. Clin. Dev. 2020; 18: 803–10. . Physical titer determination is an integral component of process monitoring from culture growth to downstream purification, and for quality control (QC) testing of product attributes of potency and purity. These assays may be included as critical quality attributes (CQAs) identified by the manufacturer as “a physical, chemical, biological, or microbiological property or characteristic that should be within an appropriate limit, range, or distribution to ensure the desired product quality” as defined by the FDA [9]Guidance for Industry Q8(R2) Pharmaceutical Development. 2009: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm.
The need for improved accuracy, speed & low volumes
Improvements in analytical techniques, specifically in analysis speed, accuracy, and sample volume consumption, have been identified as a target for helping to meet compressed timeline demands for the production of viral vectors [10]le Bec C. Priorities for AAV vector analytics development. Cell Gene Ther. Ins. 2020; 6(6): 871–5. le Bec C. Priorities for AAV vector analytics development. Cell Gene Ther. Ins. 2020; 6(6): 871–5. le Bec C. Priorities for AAV vector analytics development. Cell Gene Ther. Ins. 2020; 6(6): 871–5. , [11]Viral Vector Characterization: A Look at Analytical Tools – Cell Culture Dish: https://cellculturedish.com/viral-vector-characterization-analytical-tools/, [12]Espejo A. The right analytical toolbox is key to moving your pipeline forward. Cell Gene Ther. Ins. 2020; 6(7): 1029–33. .
Data quality
As the number of clinical studies for AAV and LV-based gene therapies grows, the FDA increasingly emphasizes the importance of vector titer assay reproducibility and the measurement of full:empty AAV capsid ratios to facilitate dose comparison between clinical programs. In a recent workshop, a target of ≤15% precision for measurement of empty AAV capsids was set as reasonable starting with early phase studies in order to compare clinical study efficacy and adverse events between studies. The discussion during the workshop indicated that improvements in the reliability of analytical methods for viral vector titer or a switch to newer technologies may be needed to reach this goal [10]le Bec C. Priorities for AAV vector analytics development. Cell Gene Ther. Ins. 2020; 6(6): 871–5. le Bec C. Priorities for AAV vector analytics development. Cell Gene Ther. Ins. 2020; 6(6): 871–5. le Bec C. Priorities for AAV vector analytics development. Cell Gene Ther. Ins. 2020; 6(6): 871–5. , [13]Quantitation of AAV-Based Gene Therapy Products – 12/07/2018 – 12/07/2018 | FDA: https://www.fda.gov/vaccines-blood-biologics/workshops-meetings-conferences-biologics/quantitation-aav-based-gene-therapy-products-12072018-12072018.
Speed
Compressed timelines for gene therapy manufacturing intensify the need for faster analytical approaches to characterize the therapeutic to verify quality and titer [7]Gene Therapies Push Viral Vector Production – Bioprocess Development Forum: http://www.processdevelopmentforum.com/articles/gene-therapies-push-viral-vector-production/Gene Therapies Push Viral Vector Production – Bioprocess Development Forum: http://www.processdevelopmentforum.com/articles/gene-therapies-push-viral-vector-production/. Many existing methods are time-consuming, producing results long after the time window for adjustment of growth or purification conditions has passed. Long assay times also add to the bottleneck in the development and production of new products [5]Rininger J, Fennell A, Schoukroun-Barnes L, Peterson C, Speidel J. Capacity Analysis for Viral Vector Manufacturing Is There Enough? BioProcess Int. 2019; 17(11). Rininger J, Fennell A, Schoukroun-Barnes L, Peterson C, Speidel J. Capacity Analysis for Viral Vector Manufacturing Is There Enough? BioProcess Int. 2019; 17(11). Rininger J, Fennell A, Schoukroun-Barnes L, Peterson C, Speidel J. Capacity Analysis for Viral Vector Manufacturing Is There Enough? BioProcess Int. 2019; 17(11). , [8]Transfiguracion J, Tran MY, Lanthier S et al. Rapid In-Process Monitoring of Lentiviral Vector Particles by High-Performance Liquid Chromatography. Mol. Ther. Meth. Clin. Dev. 2020; 18: 803–10. Transfiguracion J, Tran MY, Lanthier S et al. Rapid In-Process Monitoring of Lentiviral Vector Particles by High-Performance Liquid Chromatography. Mol. Ther. Meth. Clin. Dev. 2020; 18: 803–10. .
Sample volumes
Regulatory demands have increased the number of analyses required for characterization, putting an even higher premium on analytical techniques requiring less sample. While suspension-adapted cultures of HEK293 or insect cell (Sf9) baculovirus expression system cultures facilitate process scale-up, the complex process of 3-plasmid transient transfection (AAV rep and cap genes, adenovirus helper genes, and therapeutic transgene) continues to hinder batch size, batch yields of under 100 mg of virus typical [14]Besir HB, Holzinger D. The Challenges of AAV-Based Gene Therapy. European Biopharmaceutical Review. 2021; 30–2: http://www.samedanltd.com/magazine/current/12. It was estimated by one CMC specialist that almost half of the viral vector production batch may be consumed during QC bioanalysis steps [10]le Bec C. Priorities for AAV vector analytics development. Cell Gene Ther. Ins. 2020; 6(6): 871–5. le Bec C. Priorities for AAV vector analytics development. Cell Gene Ther. Ins. 2020; 6(6): 871–5. le Bec C. Priorities for AAV vector analytics development. Cell Gene Ther. Ins. 2020; 6(6): 871–5. . Any increases in viral vector production for preclinical or clinical studies will be a noticeable improvement.
Meeting immunoassay challenges with (centrifugal) force
Immunoassays have long been an integral component of preclinical and clinical biotherapeutic development, as a well-established method for detection and quantitation of antibody-based therapies in pharmacokinetic (PK) and anti-drug antibody (ADA) immunogenicity. Immunoassays are also routinely used analytical assays during vector manufacturing and bioprocessing for titer, empty/full capsid ratio, and process impurity analysis [15]Moleirinho MG, Silva RJS, Alves PM, Carrondo MJT, Peixoto C. Current challenges in biotherapeutic particles manufacturing Current challenges in biotherapeutic particles manufacturing. Exp. Opin. Biol. Ther. 2019; 451–69.. Traditional plate-based immunoassay formats such as ELISAs are fraught with drawbacks of extensive manual manipulations, long incubation times, and high sample and reagent usage all stemming from the assay design – adhering the capture reagent to the bottom of flat well microplates and the addition or removal of assay reagents and wash buffers to or from the top of the well.
Immunoassay technologies that go beyond the microplate look to address these drawbacks. A microfluidic, CD-based immunoassay format that uses centrifugal force to precisely control the flow of sample and reagents to automate assay steps has been notably effective in accelerating bioanalysis of antibody-based therapeutics. The microfluidic technology integrated in Gyrolab® platform (Gyros Protein Technologies) with 96 or 112 flow through streptavidin-coated bead-based affinity columns for parallel assay automation, eliminates the need for lengthy incubation times (Figure 1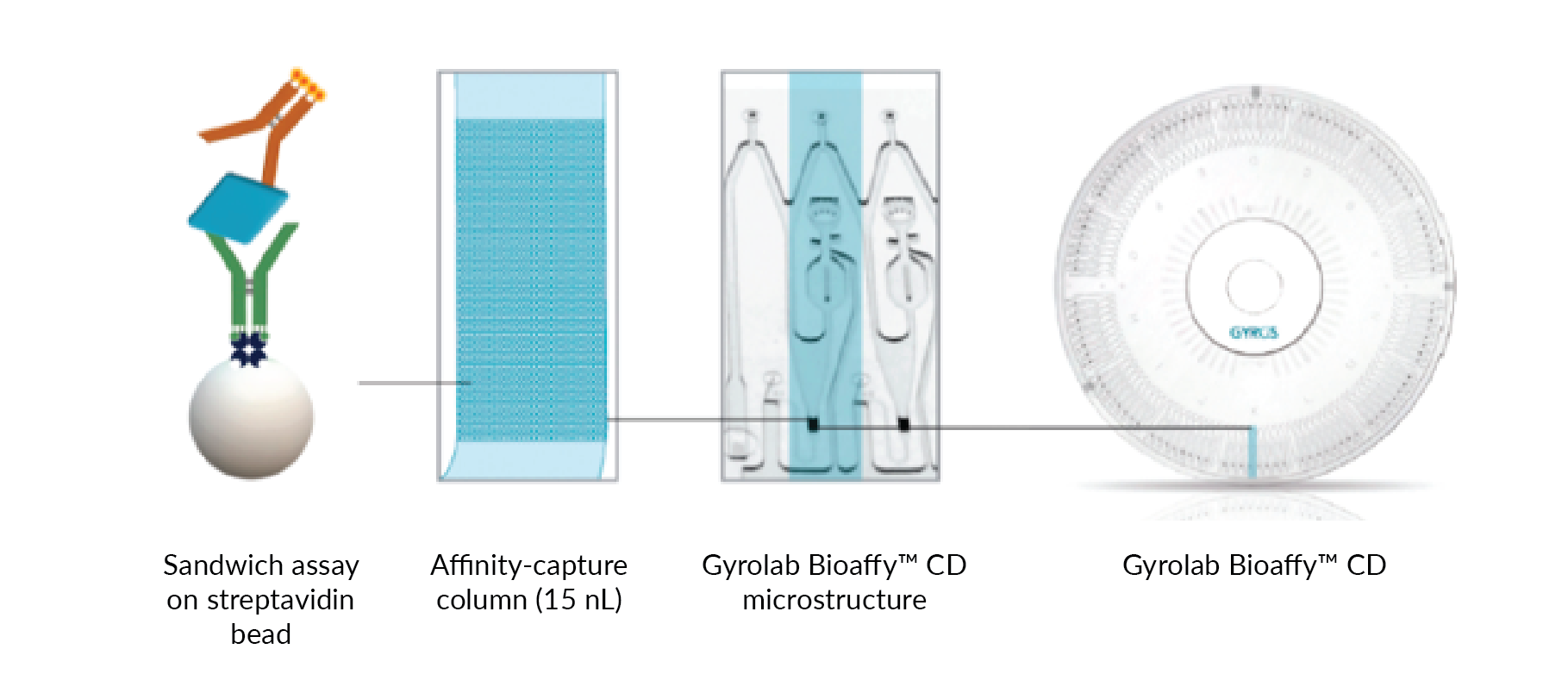
This automated, microfluidic format with an affinity flow-through column format facilitates high binding capacity, resulting in a large dynamic range and shortens sample-contact time, minimizing assay susceptibility to matrix interference. These substantial improvements in immunoassay speed (4x faster), dynamic range (1-2 log expansion of dynamic range), and sample volume (20x less) are summarized in Table 1.
| Table 1 Performance of Gyrolab® AAVX capsid titer immunoassay exceeds ELISA performance and suitability for bioprocessing development. | ||
| ELISA | Gyrolab | |
| Sample volume required | 100-200 µL | 8 µL |
| Number of hands-on steps | 5 | 1 |
| Total assay time | 4 hours | 1 hour |
| Dynamic range | 1-2 logs | >3 logs |
| When compared to ELISA kits, Gyrolab microfluidic immunoassays greatly reduce the sample volumes, hands-on time required, and overall assay time, while expanding the assay dynamic range. These dramatic improvements in assay performance and sample consumption meet the demands for advances in vector titer bioanalysis required by gene therapy compressed production timelines and limitations on batch yields. | ||
Viral vector titer analysis: breaking barriers
Total LV vector titer is typically monitored in-process during production and purification steps by quantitation of free p24 antigen, a component of the LV capsid (Figure 2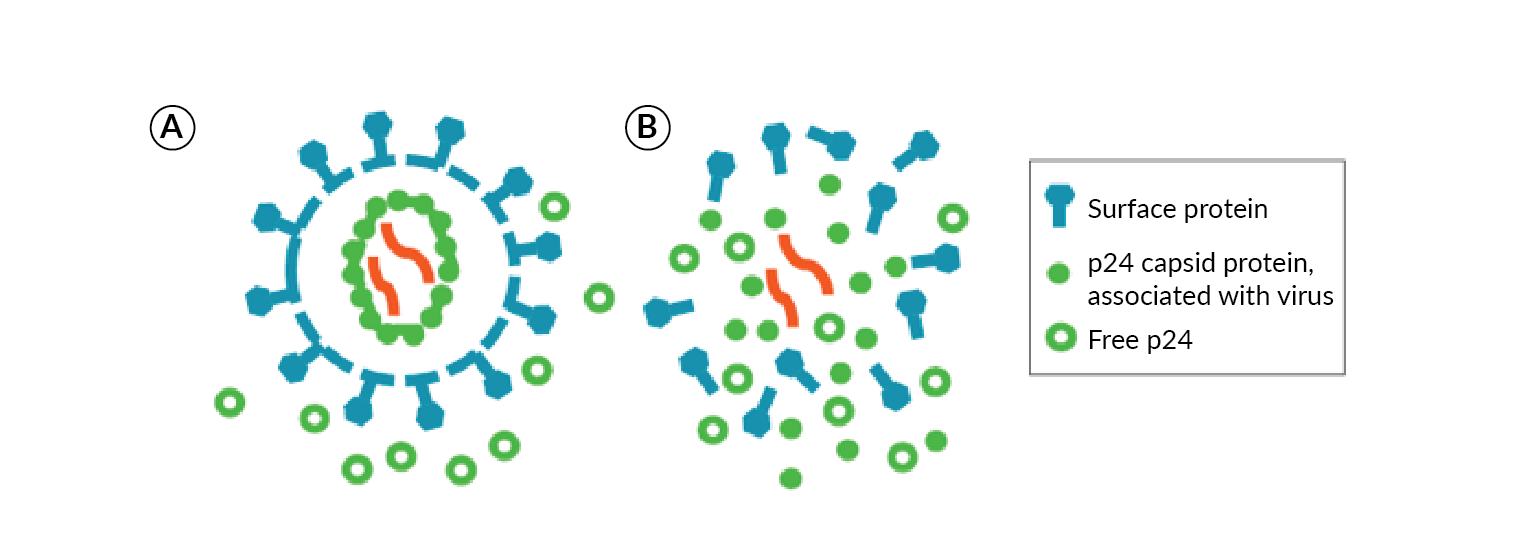
| Table 2 Intra- and inter-run precision for the Gyrolab® p24 Titer Kit standard curve samples. | ||||
| Expected conc (ng/mL) | Average measured conc (ng/mL) | Intra-run1 CV (%) | Inter-run2 CV (%) | |
| Blank | 0 | |||
| Standard 13 | 1250 | 1250 | 3.6 | 3.1 |
| Standard 2 | 250 | 251 | 2.3 | 1.9 |
| Standard 3 | 50 | 50 | 2.8 | 2.7 |
| Standard 4 | 10 | 10 | 2.9 | 2.8 |
| Standard 5 | 2 | 2 | 1.7 | 2.1 |
| Standard 6 | 0.4 | 0.4 | 2.0 | 1.8 |
| Standard 7 | 0.08 | 0.08 | 5.0 | 5.3 |
| Data for standard curve samples over the assay working range were run in duplicate in six runs on four instruments by three operators. (Six duplicate runs were performed on four different instruments, or N=12 per standard concentration). The intra- and inter-run precision was well under 10% (1.7–5.3%), demonstrating an extremely robust assay. The microfluidic design, flow-through affinity column, and automated assay all contribute to the reproducibility of assay results from run to run. 1Intra-run CV (%) = standard deviation of response divided by mean response from one run performed in duplicates. 2Inter-run CV (%) = standard deviation of means from six runs performed in duplicates divided by mean response for the six runs. 3Purified recombinant p24 standards diluted in assay buffer. | ||||
Quantitation of AAV vectors during downstream processing can be complicated by the presence of empty and partially filled capsids and the need for serotype-specific assays [17]Hajba L, Guttman A. Recent Advances in the Analysis Full/Empty Capsid Ratio and Genome Integrity of Adeno-associated Virus (AAV) Gene Delivery Vectors. Curr. Mol. Med. 2021; 20(10): 806–13. . Several analytical methods are routinely used for vector quantitation, all with distinctive drawbacks. The most widely used, quantitative real-time polymerase chain reaction (qPCR) is hampered by amplification inefficiency, inhibitors, and a standard curve requirement [18]François A, Bouzelha M, Lecomte E et al. Accurate Titration of Infectious AAV Particles Requires Measurement of Biologically Active Vector Genomes and Suitable Controls. Mol. Ther. Meth. Clin. Dev. 2018; 10: 223–36. although the increasing adoption of droplet digital PCR (ddPCR) eliminates the standard curve requirement and is less subject to sample inhibition and amplification inefficiency. ELISAs detecting assembled AAV capsid proteins (of various serotypes) are commonly used for total AAV capsid quantitation but have the typical plate-based assay drawbacks of narrow dynamic range, long assay times, and involve many manual interventions.
AAV total capsid titer quantitation using the microfluidic format Gyrolab AAVX Titer Kit immunoassay has been shown to significantly improve data quality over plate-based methods by expanding the dynamic range, from 1.5 logs for a commercially available ELISA AAV2 kit to 3 logs for the Gyrolab AAVX Titer Kit (Figure 3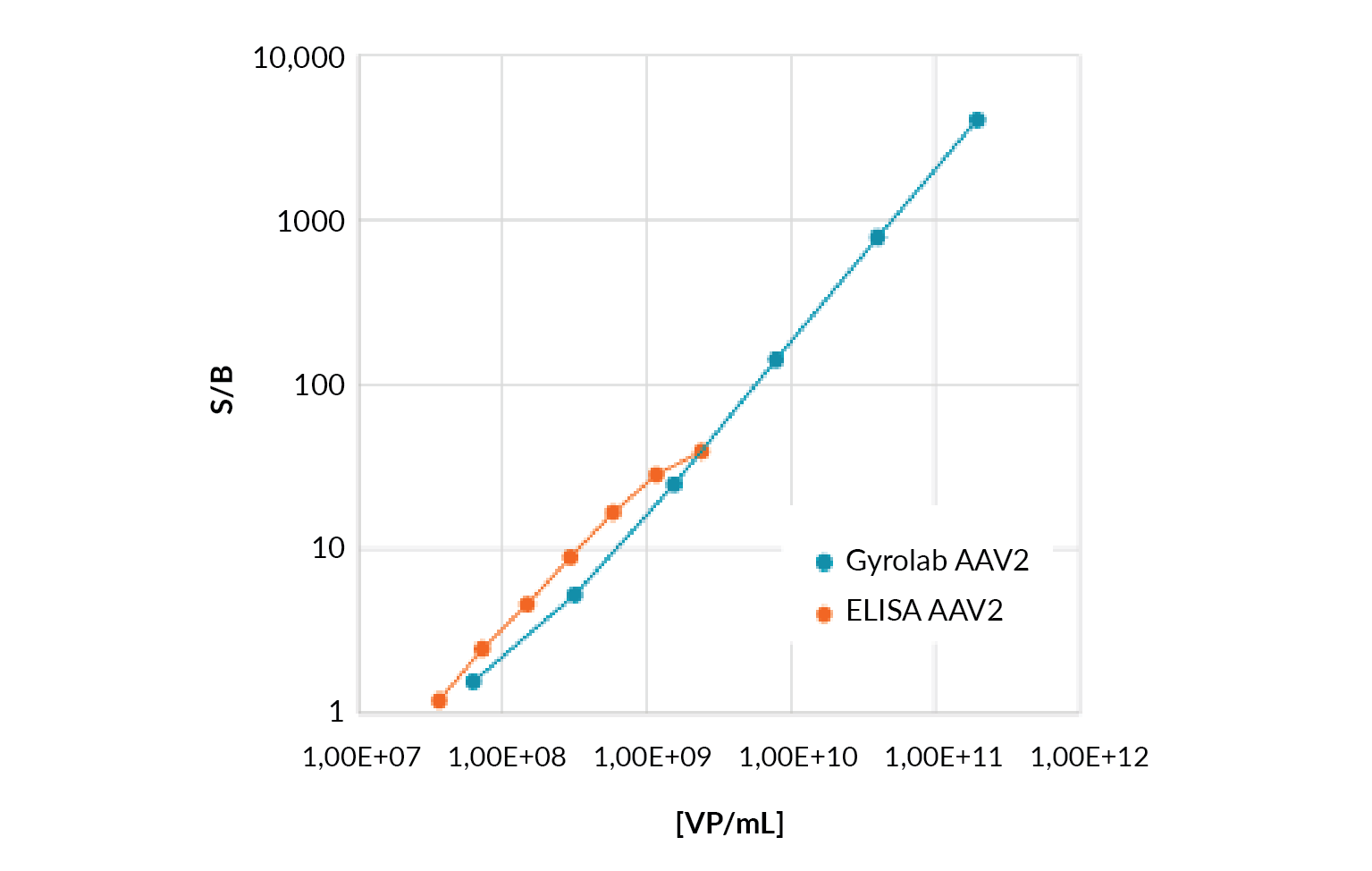
Gyrolab AAVX Titer Kits provide a single reagent set solution for most AAV serotypes, compared to the need for different detection reagents for each serotype in commercially available ELISA kits. Resolving the need to source multiple detection antibodies for different AAV serotypes, the Gyrolab AAVX Titer Kit incorporates Thermo Scientific™ CaptureSelect™ anti-AAVX (ThermoFisher Scientific) ligand that has binding selectivity and affinity for a range of AAV serotypes, with specificity towards assembled capsids. The biotinylated CaptureSelect anti-AAVX ligand as capture reagent and Alexa Fluor® 647-labeled CaptureSelect anti-AAVX as detection reagent was shown to be suitable for quantitation of AAV serotypes 1–8 and rh10 (Figure 4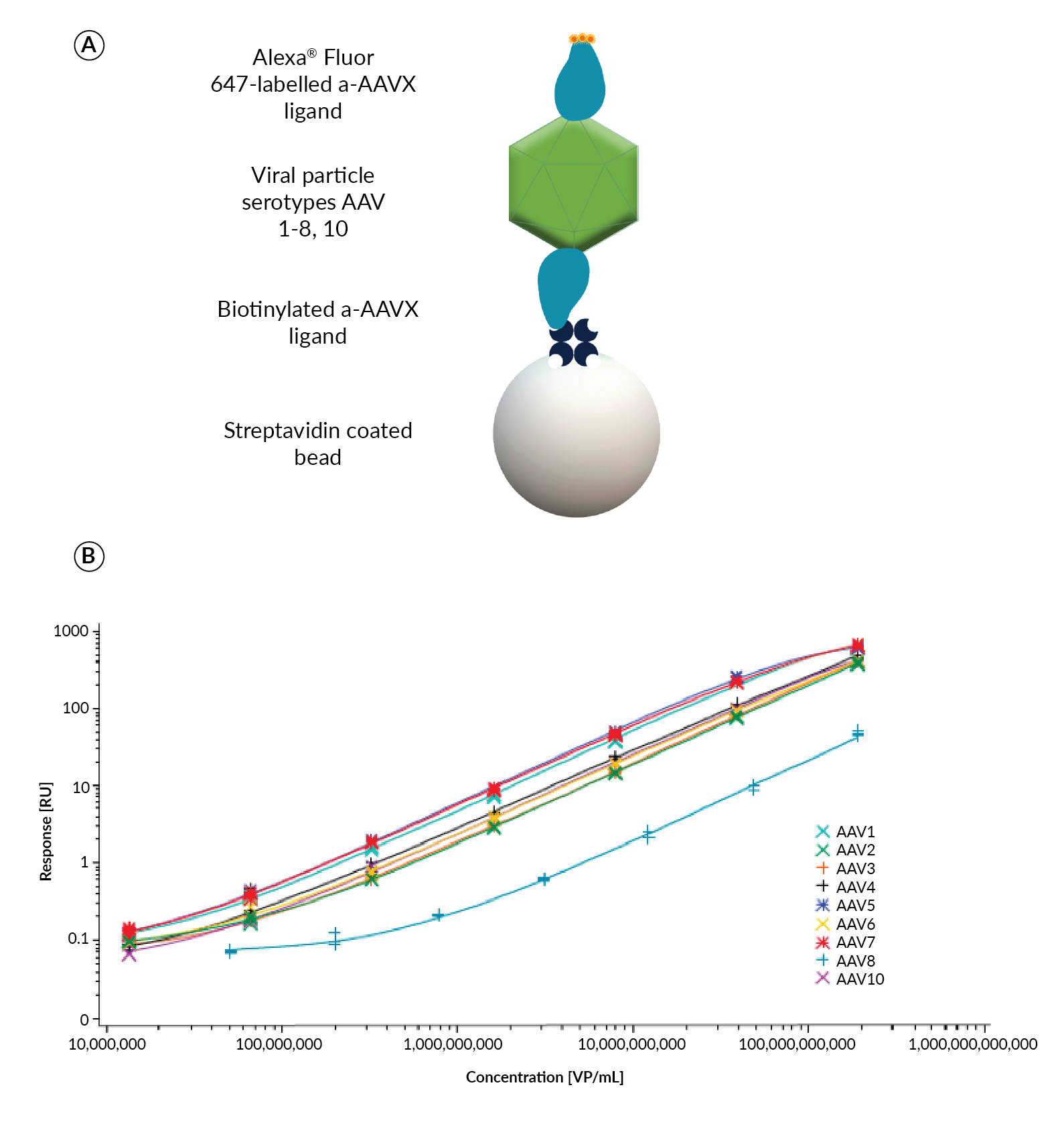
Combining qPCR or ddPCR techniques to measure vector genomes with ELISA for total capsid titer to determine AAV full-to-empty capsid ratios has been shown to be an effective and higher throughput method over transmission electron microscopy (TEM) and analytical ultracentrifugation [22]Fu X, Chen W-C, Argento C et al. Analytical Strategies for Quantification of Adeno-Associated Virus Empty Capsids to Support Process Development. Hum. Gene Ther. Meth. 2019; 30(4): 144–52. although ELISA has generally been considered to be an imprecise approach and limited to single AAV serotype assays. Gyrolab immunoassays for total capsid titer overcome these limitations can provide complete and rapid quantification of full-to-empty capsid ratios for accurate titer analysis, enabling data-driven decision-making relevant to production and bioprocess timelines.
Impurity analysis where speed & data quality count
Process-related impurities present in biologics are highly regulated because of their immunogenic potential and associated risks to product safety, efficacy, and quality. Assays for measuring complex heterogeneous mixtures of culture-related host-cell proteins (HCPs) need to be robust and reproducible to meet regulatory requirements for analysis of samples throughout manufacturing and bioprocessing.
Human embryonic kidney (HEK) 293 or HEK 293T adherent or suspension cell lines often are used to produce AAV and LV vectors for cell and gene therapies and for viral vector vaccines. ELISAs are the most commonly used method for HCP characterization of HEK 293 AAV or LV bioprocess samples but have drawbacks of high assay variability and low productivity. Thus, repeat analyses are common, delaying project decisions and lot approvals. Gyrolab immunoassays in a sandwich assay format using anti-HEK 293 HCP antibodies (Cygnus Technologies) as capture and detection reagents have been developed, delivering data for HCP analysis over a broad working range of 2–10,000 ng/mL. The broad assay range minimizes sample repeats, and the 1-hour assay time allows higher throughput analyses to keep up with the large number of samples generated during bioprocessing, with up to 960 datapoints/day.
Summary
Improvements in bioanalysis approaches are urgently needed to meet the demands of compressed development timelines for gene therapies and backlogs in manufacturing pipelines. Immunoassays, traditionally using plate-based methods, are an attractive target for improvements in assay time, dynamic range, and sample volume requirements. Significant advances in these areas have been made by the microfluidic, nanoliter-scale Gyrolab immunoassay platform, producing assay data in one hour with a wide dynamic range, and consuming under 10 µL of sample. These dramatic immunoassay improvements are expected to alleviate the bioanalysis timeline delays in viral vector manufacturing.
References
1. Lapteva L, Purohit-Sheth T, Serabian M, Puri RK. Clinical Development of Gene Therapies: The First Three Decades and Counting. Mol. Ther. Meth. Clin. Dev. 2020; 19: 387–97. Crossref
2. Ma CC, Wang ZL, Xu T, He ZY, Wei YQ. The approved gene therapy drugs worldwide: from 1998 to 2019. Biotechnol. Adv. 2020; 40: 107502.
3. Medicines in Development for Cell and Gene Therapy 2020; 2020.
4. Maldonado R, Jalil S, Wartiovaara K. Curative gene therapies for rare diseases. J. Commun. Genet. 2020: 1–10. Crossref
5. Rininger J, Fennell A, Schoukroun-Barnes L, Peterson C, Speidel J. Capacity Analysis for Viral Vector Manufacturing Is There Enough? BioProcess Int. 2019; 17(11). Crossref
6. Quinn C, Young C, Thomas J, Trusheim M. Estimating the Clinical Pipeline of Cell and Gene Therapies and Their Potential Economic Impact on the US Healthcare System. Value in Health 2019; 22(6): 621–6.
7. Gene Therapies Push Viral Vector Production – Bioprocess Development Forum: http://www.processdevelopmentforum.com/articles/gene-therapies-push-viral-vector-production/ Crossref
8. Transfiguracion J, Tran MY, Lanthier S et al. Rapid In-Process Monitoring of Lentiviral Vector Particles by High-Performance Liquid Chromatography. Mol. Ther. Meth. Clin. Dev. 2020; 18: 803–10. Crossref
9. Guidance for Industry Q8(R2) Pharmaceutical Development. 2009: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm Crossref
10. le Bec C. Priorities for AAV vector analytics development. Cell Gene Ther. Ins. 2020; 6(6): 871–5. Crossref
11. Viral Vector Characterization: A Look at Analytical Tools – Cell Culture Dish: https://cellculturedish.com/viral-vector-characterization-analytical-tools/ Crossref
12. Espejo A. The right analytical toolbox is key to moving your pipeline forward. Cell Gene Ther. Ins. 2020; 6(7): 1029–33. Crossref
13. Quantitation of AAV-Based Gene Therapy Products – 12/07/2018 – 12/07/2018 | FDA: https://www.fda.gov/vaccines-blood-biologics/workshops-meetings-conferences-biologics/quantitation-aav-based-gene-therapy-products-12072018-12072018 Crossref
14. Besir HB, Holzinger D. The Challenges of AAV-Based Gene Therapy. European Biopharmaceutical Review. 2021; 30–2: http://www.samedanltd.com/magazine/current/12 Crossref
15. Moleirinho MG, Silva RJS, Alves PM, Carrondo MJT, Peixoto C. Current challenges in biotherapeutic particles manufacturing Current challenges in biotherapeutic particles manufacturing. Exp. Opin. Biol. Ther. 2019; 451–69. Crossref
16. Gyrolab® P24 Kit. Product Information Sheet: https://www.gyrosproteintechnologies.com/gyrolab-p24-kit Crossref
17. Hajba L, Guttman A. Recent Advances in the Analysis Full/Empty Capsid Ratio and Genome Integrity of Adeno-associated Virus (AAV) Gene Delivery Vectors. Curr. Mol. Med. 2021; 20(10): 806–13. Crossref
18. François A, Bouzelha M, Lecomte E et al. Accurate Titration of Infectious AAV Particles Requires Measurement of Biologically Active Vector Genomes and Suitable Controls. Mol. Ther. Meth. Clin. Dev. 2018; 10: 223–36. Crossref
19. Gyrolab® AAVX Titer Kit: https://www.gyrosproteintechnologies.com/gyrolab-aavx-titer-kit Crossref
20. Petrovic S, Fromentin Y, Pelluet P et al. Host-Cell Protein Analysis to Support Downstream Process Development A High-Throughput Platform with Automated Sample Preparation. Crossref
21. Case Study: Evaluation of Progen Adeno-Associated Virus Assay on Gyrolab Immunoassay System: https://page.gyrosproteintechnologies.com/evaluation-of-progen-adeno-associated-virus-assay-on-gyrolab-system-case-study Crossref
22. Fu X, Chen W-C, Argento C et al. Analytical Strategies for Quantification of Adeno-Associated Virus Empty Capsids to Support Process Development. Hum. Gene Ther. Meth. 2019; 30(4): 144–52. Crossref
Affiliations
Patricia Ahrweiler
Gyros Protein Technologies
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: Many thanks to Daniel Forsström, Hannah Litwin and Lena Lilja for providing product performance data.
Disclosure and potential conflicts of interest: The author is an employee of Gyros Protein Technologies.
Funding declaration: The author received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2021 Gyros Protein Technologies. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited.
Article submitted: Nov 30 2020; Revised manuscript received: Mar 1 2021; Publication date: Mar 16 2021.

