Commercial Lessons for Clinical Success
Cell Gene Therapy Insights 2018; 4(11), 1131-1138.
10.18609/cgti.2018.107
Advanced therapies require highly-sophisticated advance planning. For long-term success, transformative cell and gene therapies rely on a risk-based understanding of the supply chain, with all aspects mapped out early. Recent draft guidance from regulatory authorities stresses early end-to-end systems thinking and data management capabilities. A commercially-minded approach to each clinical phase will help create viable commercial supply chains and patient journeys from the beginning. This article provides valuable commercial-phase insights from industry veterans with deep commercialization experience, and discusses how these lessons can be applied to clinical-phase therapies to help drive advancement and success.
In the offices of an early-stage biopharmaceutical developer, the team has received exciting news. Their early-phase advanced therapy trial, currently operating at just one clinical site, has earned investigators’ interest and will expand faster than planned.
The Chief Medical Officer hopes to expand the trial to four clinical sites within 10 weeks. The initial site, located in a major city with two airports, runs smoothly. Leukapheresis takes place in the morning, allowing for rapid transfer of collected patient material to a nearby airport within hours. The clinical site is geographically close to the manufacturing site, meaning that the patients’ cells are shipped to manufacturing overnight. Traceability and logistics have been run manually. Three patients have been treated, with a fourth patient journey in process.
This initial success raises the team’s optimism as they consider the new clinical sites —two based in major metropolitan areas much farther away, and a third in a more rural location with one smaller, remote airport. There, leukapheresis will be performed at a local blood bank, not at the clinical site.
The therapy continues to change patients’ lives. But as the trial tries to grow, operations falter, and the therapy never reaches patients at commercial scale. Why?
The company had secured funding, trained clinicians, and managed costs. But as the trial grew, its operators did not account for the complexity that scale brings. Moving therapies through multiple airports, time zones, hospital systems—plus the tracking challenges that emerged—became daunting. An efficacious and safe therapy couldn’t reach more patients because the company had not understood the criticality of building a digitally scalable, traceable logistics platform.
| KEY TERMS AND CONCEPTS |
Several key terms are central to this discussion:Chain of Identity (COI): The permanent and transparent association of an autologous donor’s unique identifiers to their tissue and/or cells from order through collection, manufacturing, administration, and post treatment monitoringChain of Custody (COC): Permanent data capture from the start of tissue and/or cell collection through to product administration of the staff that handled the product, actions performed by those staff, and the location/date/time of those actions Chain of Condition (COCn): Permanent data capture from the start of tissue and/or cell collection through to infusion that tracks temperature and other key variables essential to the quality and viability of cells and finished product Logistics Platform: The repeatable, scalable systems used to securely execute a therapy’s critical shipments (inclusive of donation, vector, therapy, and monitoring samples) with full alignment to supporting COI, COC and COCn. |
Critical lesson: start with the end state in mind
For long-term success, advanced therapies require a risk-based understanding of the supply chain with all aspects mapped early. Recent draft guidance from the U.S Food and Drug Administration on the use of expansion cohorts in first-in-human clinical trials for oncology drugs and biologics stresses early end-to-end systems thinking and data management capabilities. The draft guidance states [1]U.S. Food and Drug Administration, Expansion Cohorts: Use in First-In-Human Clinical Trials to Expedite Development of Oncology Drugs and Biologics Guidance for Industry, August 2018. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM616325.pdf:
“To… protect patients, it is imperative that sponsors establish an infrastructure to streamline trial logistics, facilitate data collection and incorporate plans to rapidly assess emerging data in real time and to disseminate interim results to investigators, institutional review boards (IRBs), and regulators.”
| Phase 0 | Phase I/II | Phase II+ | Phase III | Phase IV | |
|---|---|---|---|---|---|
| ENVISION | Build | Operate | Optimize | Grow | |
| This very active phase precedes an Investigational New Drug (IND) application, and in oncology-focused advanced therapies, increasingly involves activities once more common in later phases. First-in-human trial designs are drawing increasing interest and may require pre-IND planning to prepare for larger early-phase patient cohorts. [3]U.S. Food and Drug Administration, August 2018. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM616325.pdf Protocols in such trials may be designed to develop and manage a baseline of patient safety data using existing patient-based best practices, rather than novel animal model data. As early clinical sites are being assessed and arranged, investigators may bring existing knowledge to the “Envision” phase. These sites may already be familiar with existing advanced therapies systems that support COI, COC, and COCn, and prefer to leverage known processes and partners as much as possible. Traditionally, activities and planning for logistics, digital supply chain management and other enabling technologies are more limited in this phase. However, as advanced therapy developers acknowledge new early-phase complexities, we find that an increasing number are doing more systems planning during “Envision” and including discussion of these early preparations in their IND filings. | In oncology-focused advanced therapies, this bundled phase often precedes a pivotal trial, and may require initiating a variety of activities quickly. If a first-in-human, multi-expansion cohort trial design is being pursued, the size of the patient cohort may grow significantly, even at this early stage. [4]U.S. Food and Drug Administration, August 2018. Some of the activities in this “Build” phase are common in biopharmaceutical development, such as focus on scientific proof of concept and definition of the therapeutic product. Other activities reflect unique factors in advanced therapies. Through mechanisms such as the U.S. FDA’s Regenerative Medicine Advanced Therapy Designation (RMAT), regulatory pathways and timelines may also be accelerated [5]U.S Food and Drug Administration, Regenerative Medicine Advanced Therapy Designation, February 2018. https://www.fda.gov/biologicsbloodvaccines/cellulargenetherapyproducts/ucm537670.htm. With high expectations from clinical partners, regulators and investors, speed is essential. Supporting systems that enable the continuous collaboration required in advanced therapies take on increased importance, and need to grow and adjust as lessons are learned in real-time. In the “Build” phase, activities and planning for logistics platforms, digital supply chain management and other enabling technologies reflect this need for speed. While these systems may not yet need to scale out widely, they do need to support accelerated timelines as well as high expectations from clinical site investigators, Quality teams, investors and regulatory experts. | In oncology-focused advanced therapies, this “Operate” phase often involves a pivotal trial. Patient volume will continue to grow, and the number of clinical sites may also expand internationally. This phase offers an ideal time to actively prepare for commercialization and establish the underlying systems and protocols that will enable safety and efficiency at scale. For example, growing patient and site volumes often require active management of manufacturing capacity. This same growth often requires expanded logistics and supply chain management systems, ensuring that these systems have the flexibility to integrate both internally and externally. Amid this growth, biopharmaceutical developers may face pressure to begin locking their protocols, systems and SOPs, including those related to logistics and supply chain, as part of preparation for a regulatory application filing. In the “Operate” phase, advance work on logistics platforms, digital supply chain management and other enabling systems repeatedly demonstrates a return on investment. If the necessary foundation has been designed thoughtfully and laid in advance, this phase points the way towards a fully-developed vision of commercial success. | This phase is the “dress rehearsal” for commercial launch. In the accelerated world of advanced therapies, this phase may sometimes be combined with Phase II ½ “Build.” A range of stakeholders, including clinical investigators, regulators, and investors, expect visibility into a wide range of data, from expanded efficacy endpoints to systems’ operations. Patient volume may continue to expand, with corresponding growth in complexity. At the “Optimize” phase, underlying systems and processes, including those related to logistics and supply chain, will be tested, validated and then enter a semi-locked demonstration state for regulatory filings. Demonstrating mastery of COI/COC and COCn will be an essential part of the biologics application. [6]U.S. Food and Drug Administration, August 2018. The “Optimize” phase also provides invaluable data that shows how the patient journey will work in the commercial world. Data management takes on new importance. Regulators are likely to require a complete data download of key actions that occurred on behalf of each “intent to treat” patient. Commercial teams will require data as they ramp up for a launch that will rely on order-to-cash and reimbursement processes, post-approval. In this phase, logistics systems, digital supply chain management and other enabling technologies should now be running in real time. Logistics is now a key concern and a fully formed logistics platform is required. A documented, validated digital supply chain management system is also a requirement, and internal integrations between a full range of e-systems should now be underway. | Even after commercial launch, a wide variety of stakeholders, including healthcare providers and investors, expect continuous expansion and improvement, testing underlying SOPs and systems in new ways. Customer service, with a constant need for data and transparency from order to delivery, becomes critical. Regulators and payers may present requirements for long-term patient monitoring, extending the patient journey years beyond treatment. If launch is successful, scaling up and out continues. A commercialized advanced therapy must contend with both legacy and new competitors for healthcare provider and patient interest and continue to demonstrate value. Understanding and controlling COGS takes on higher priority. At this “Grow” point, certain aspects of the underlying systems and process such as manufacturing, are standardized and validated, and therefore somewhat fixed. However, logistics platforms and digital supply chain management systems can continue to evolve within approved regulatory parameters—and be used as points of differentiation in an ever more competitive market. | |
| Highlights and takeaways | |||||
 Focus Focus | Your IND | Safety and initial efficacy | Proof of principles (all of them) | Efficacy at scale, preparing for filing and launch | Can I do more of this, for less? |
 Big Question Big Question | Are you patient ready? | When to automate, how to scale? | Can this work in Commercial? | Can I file and launch quickly and smoothly? | Can I manage commercial and clinical all at once? |
 Lesson 1 Lesson 1 | Start as you mean to continue, even now. | Plan systems and processes for the future | Get real-time feedback on your process | Be ready to show how you will operate in the market. This impacts process development and enables investors and regulators to support the therapy. | Scaling becomes multi-dimensional |
 Lesson 2 Lesson 2 | Take your traceability and logistics platform as seriously as your science. | Create real, scalable integrations throughout the supply chain. | Develop the capability to operate logistics platform, COI/COC and COCn at volume | COGS improvement requirements will start to kick in. | New collaborations keep emerging. |
 Lesson 3 Lesson 3 | Regulators will expect solid systems, even early on. | Seek out flexibility | Ensure quality and compliance now, in ways that can scale efficiently | Regulators’ expectations for numerous systems, including COI/COC, are increasingly set. | Success begets more demand, which will continue to pressure-test systems and processes. |
 Expert Experttip | Use Target Product Profiles to identify critical logistics attributes and assess their impact as the therapy scales. This produces a development pathway in-line with process development and gives confidence to investors that the therapy is a viable commercial proposition. | Work with proven logistics and supply chain partners to think proactively about workflows and apply proven solutions. Use critical parameters to start overcoming critical supply chain and logistics bottlenecks early, addressing the highest risks first. Design for scale, even if part of the process remains manual for now. Starting with some automation and keeping integrations simple will allow room for learning | Don’t underestimate the importance of this phase. While not necessarily part of traditional clinical phasing, this “Operate” period lays critical early groundwork for commercial- scale traceability and logistics platforms. This is a critical time for establishing scalable processes and systems to support commercialization—and patients. | Finalize your logistics platform and use that template to smoothly transfer into new geographies. Manage complexity from less experienced sites that will therefore need more support, placing a greater burden on the therapy developer. | Logistics can be a key differentiator and provide additional patient and provider value. Logistics and traceability platforms should be constantly reviewed and take internal and external changes into account, such as shipping volumes or political events. |
As three pioneering leaders in advanced therapy operations and delivery, we present commercially-minded thinking on the use of clinical phases to create viable commercial supply chains and patient journeys from the beginning. While these lessons apply to a wide variety of advanced therapy indications, this article will focus most directly on oncology, currently the largest single indication being pursued [2]Alliance for Regenerative Medicine, Quarterly Data Report, November 1, 2018. http://alliancerm.org/wp-content/uploads/2018/10/ARM_Q3_2018_Web-1.pdf.
Development phases in advanced therapies
As part of a commercially-minded approach, it is also important to note that in biopharmaceutical development, the nature of clinical phases is changing. In advanced therapies, that change is accelerated. Increasingly, clinical phasing doesn’t conform neatly to the norms of “Phase I,” “Phase II,” “Phase III,” and “Commercial Launch.” We’ve often seen five phases emerge, rather than the traditional four, with some activities pulled forward earlier and new activities emerging. In this section, we outline our view of these five phases, with success factors for each.
Concluding insights
Advanced therapies face a pivotal moment. With more than 570 [7]Alliance for Regenerative Medicine, November 2018. oncology-focused advanced therapies in the discovery pipeline, now is the time for early-phase developers to benefit from lessons that accelerate patient journeys.
Here are final take-aways:
- Understand the evolving nature of each development phase in advanced therapies. First-in-human trial designs and expedited designations such as the EMEAs PRIME or FDA’s RMAT or Breakthrough Therapy designations present the opportunity for accelerated trial processes and may require logistics and systems thinking earlier than anticipated. The Phase II+ “Operate” period is critical, laying essential groundwork for the logistics platform, COI/COC and COCn at scale.
- Commercial success brings more innovation. A successful therapy developer may face the need to manage a commercial therapy and a pipeline of emerging clinical-phase products all at the time. Try to standardize underlying systems across all products as much as possible. While it’s common to use different systems for commercial and clinical, this bifurcation may reduce efficiency, increase costs, and introduce increased risk.
- The advanced therapies industry is increasingly comfortable with clinical and process development stages. But logistics and traceability are just as important and can have significant effects on product development and future commercialization. Consider a risk-based approach to understanding your critical logistics attributes. Aligning your logistics platform development and your clinical and manufacturing platforms will save time and cost, as well as support a more efficient transition to commercial scale.
- Above all, seek input from industry experts who have been there before. Partners who you might typically call “vendors” are actually technical experts who have overcome the challenges you face. A large number of learnings already exist in this nascent industry. Working with the right partners to plan out development, scale logistics, and properly time e-systems, automation, and integrations will be a critical factor on the journey to commercial success.
Figures
Figure 1.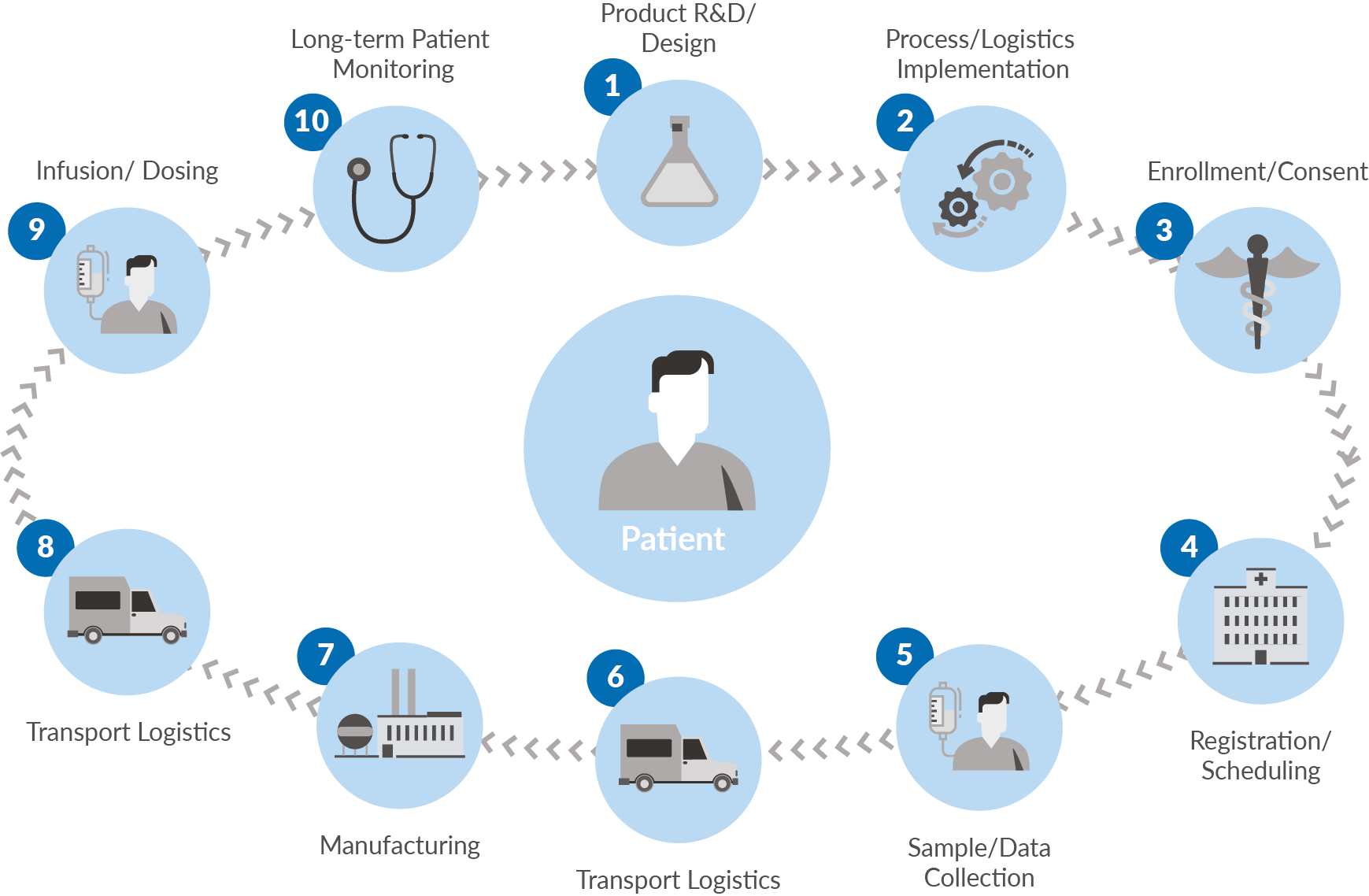
Figure 2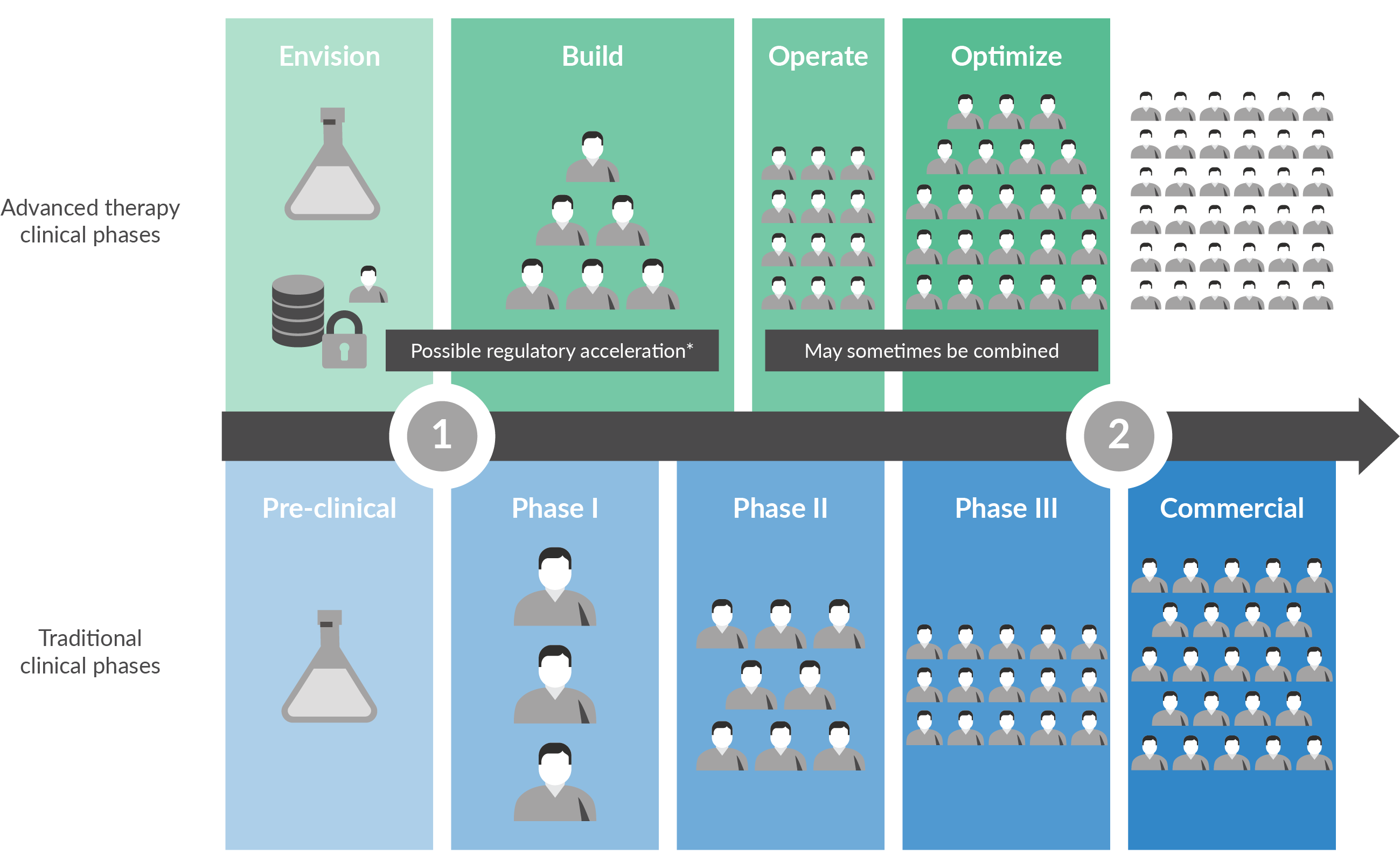
Financial & competing interests disclosure
The authors are senior leaders at Vineti Inc and World Courier. No writing assistance was utilized in the production of this manuscript.
References
1. U.S. Food and Drug Administration, Expansion Cohorts: Use in First-In-Human Clinical Trials to Expedite Development of Oncology Drugs and Biologics Guidance for Industry, August 2018. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM616325.pdf
2. Alliance for Regenerative Medicine, Quarterly Data Report, November 1, 2018. http://alliancerm.org/wp-content/uploads/2018/10/ARM_Q3_2018_Web-1.pdf
3. U.S. Food and Drug Administration, August 2018. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM616325.pdf
4. U.S. Food and Drug Administration, August 2018.
5. U.S Food and Drug Administration, Regenerative Medicine Advanced Therapy Designation, February 2018. https://www.fda.gov/biologicsbloodvaccines/cellulargenetherapyproducts/ucm537670.htm
6. U.S. Food and Drug Administration, August 2018.
7. Alliance for Regenerative Medicine, November 2018.
Affiliations
Simon Ellison, Cell and Gene Therapy Service Director, World Courier. Simon is the Cell and Gene Therapy Service Director at World Courier. His role is to develop a logistics platform that supports the growth of the advanced therapy industry through clinical development and into commercial operations.
Whilst at the Cell and Gene Therapy Catapult he identified the need for, and built the Seamless Freight portfolio. He also helped develop the commercial operating model for the Catapult Manufacturing Centre that resulted in the first collaborators entering, and the start of a cluster forming around this unique facility.
Prior to this, Simon was the Head of Commercial at NHS Blood and Transplant (NHSBT) where he merged the needs of the industry with NHSBTs manufacturing and supply chain capability. This enabled multiple UK and international organizations to move their therapies forwards.
Heidi Hagen, Chief Strategy Officer and Co-founder, Vineti Inc. Heidi has been an Operations Executive in the Biotechnology industry for over 25 years and is a Co-founder of Vineti. She has an extensive and proven track record in leading operations and commercializing innovative technologies ranging from recombinant protein/device combinations to the first active immune cell therapy, Provenge. Previously, Heidi was the Global COO for SOTIO, in Prague, Czech Republic with a US office in Boston, MA. Before joining SOTIO she worked for Dendreon for ten years as Senior Vice President of Operations and ten years with Immunex Corporation in a range of roles in drug development and operations management. Heidi has a B.S. in Cell and Molecular Biology, an MS in Bioengineering, and an MBA from the University of Washington.
Christophe Suchet, Chief Product Officer, Vineti Inc. Christophe brings more than 20 years of information technology and senior biopharmaceutical IT experience to Vineti. Most recently, he served as Vice President of IT for Kite Pharma, Inc., where he developed the essential technology systems that helped Kite’s first cell therapy receive FDA approval, scale rapidly, and secure the landmark sale of the business to Gilead Sciences, Inc. Prior to Kite Pharma, Christophe served as Vice President of IT at Pharmacyclics Inc., a clinical stage and commercial biopharmaceutical company that grew in two years from no product revenue, to $1 billion revenue, to acquisition by AbbVie for $21 billion. Christophe has served as Genentech’s Director, IT Pharma Development Applications, and as IT Director, SAP Center of Excellence & Enterprise Applications. He received his M.S. in Biology and Economy from AgroParisTech.
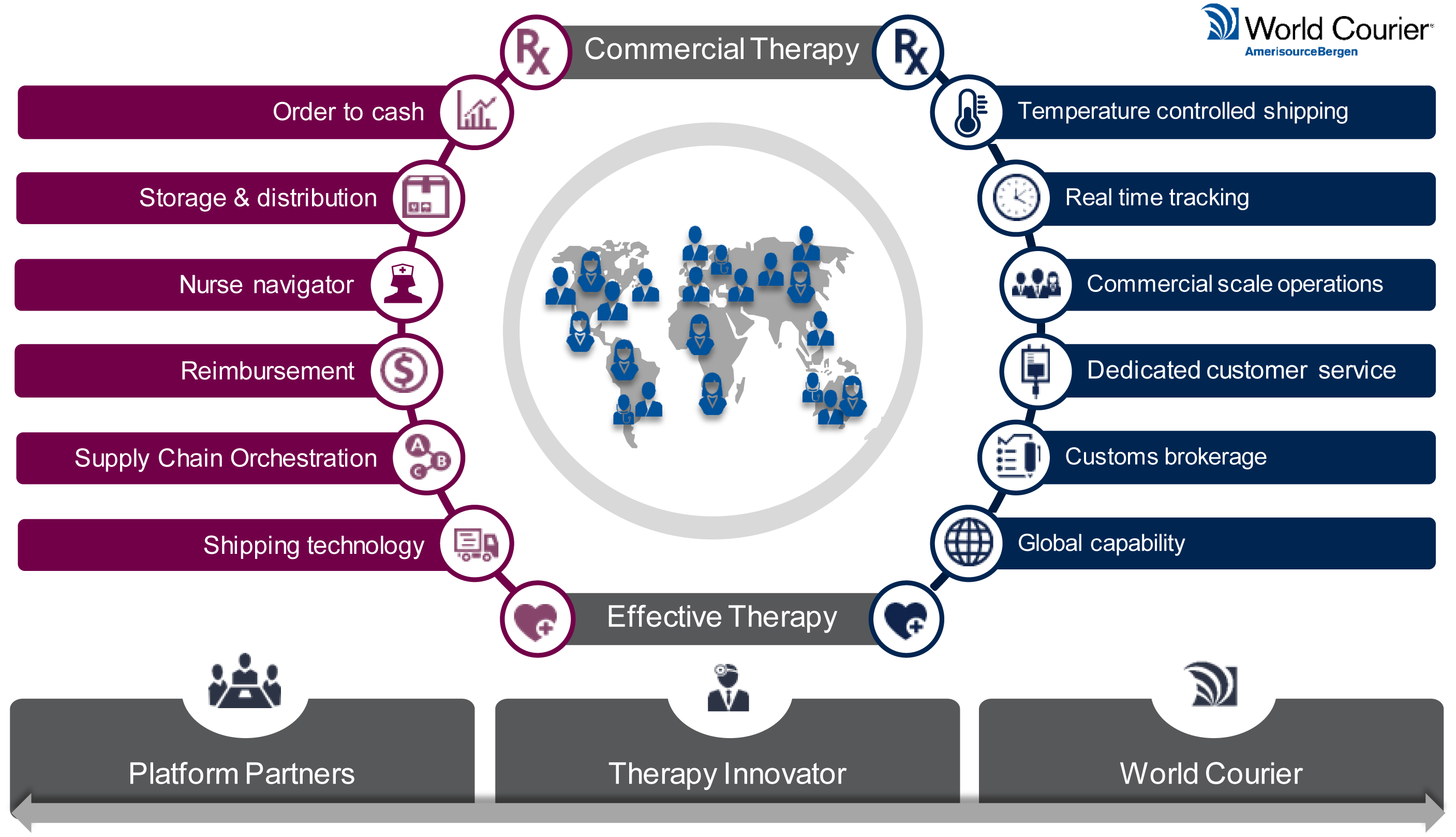 Building a logistics platform.
Building a logistics platform.