Checkpoint on I–O resistance: lessons learnt & future perspectives
Immuno-Oncology Insights 2023; 4(4), 173–192
DOI: 10.18609/ioi.2023.023
Immune checkpoint blockade via anti-PD(L)1 has revolutionized anti-cancer treatment, with durable responses observed across multiple cancer types. However, some patients are resistant to treatment and many relapse, following initial response. Here we propose a conceptual framework aimed at promoting clearer discussion and understanding of I–O resistance. Within this framework, we define two critical factors that determine the success of anti-PD(L)1 therapy. The first is visibility of the tumor, as a foreign entity recognizable by the host’s immune system. The second is T cell functionality, as an effective means of eliminating the tumor once recognized. These two core factors are subject to modification by several different tumor cell intrinsic and extrinsic aspects of biology, which are themselves interdependent. In this perspectives article, we take each of these modifiers in turn, summarizing the field’s current state of knowledge, and considering how it can be leveraged to better direct the next generation of therapies.
Since the first approval in 2014, the use of anti-PD-(L)1 based therapy has transformed the treatment of cancer. As of September 2022, anti-PD-(L)1, either alone or in combination with other therapies, had received more than 90 FDA approvals, in 20 different tumor types and in concert with three different biomarkers defined by companion diagnostics; PD-L1 expression, tumor mutational burden (TMB) and mis-match repair (MMR) deficiency. Despite this unprecedented impact on the field, the fact remains that the majority of patients still experience disease progression, and given such broad and rapid clinical success, it has been challenging for scientific understanding to keep pace. As a result, our knowledge of when and how resistance emerges remains in its infancy, and such knowledge has the potential, in the future, to help drive informed drug development that addresses resistance through combination or alternative therapy.
A significant challenge in tackling the biology of anti-PD(L)1 resistance stems from the disconnect between target engagement and effect, that is inherent to checkpoint blockade. A typical targeted oncology therapy, such as a tyrosine kinase inhibitor (TKI) or antibody drug conjugate (ADC), acts in a very direct way, binding its target and as a result mediating a, typically, cytotoxic effect on the tumor. Drivers of response and resistance to such therapies tend to be tumor intrinsic and are frequently modifiers of the target itself, either via expression or mutation, or of pathways associated with the target or the downstream cytotoxic effect, such as proton pumps or alternative oncogenic pathways that enable escape. Anti-PD(L)1, and other T cell checkpoint inhibitors such as anti-CTLA-4, mediate their anti-tumor effects indirectly. Binding to their target has no direct effect on tumor cells, but rather modulates the immune system, increasing the probability of eliciting an anti-tumor immune response. Ultimately, the immune system is the drug, and resistance can be driven by anything that modulates its effective function. The result is a complex network of interdependent response and resistance drivers that can be both tumor intrinsic and/or tumor extrinsic.
Here, we propose a conceptual framework aimed at promoting clearer discussion and understanding of immuno–oncology (I–O) resistance, which can then drive more effective use of knowledge to improve therapies. Within this framework, we define two critical features that determine the success of anti-PD(L)1 therapy, with a complex network of modifiers impacting one or both these features. We take each of these modifiers in turn, summarizing the fields current state of knowledge, considering how this knowledge might be pursued to better direct the next generation of therapies, and speculating as to where the next generation of insights may come from.
A framework for exploring anti-PD-(L)1 resistance
Anti-PD-(L)1 therapy relies upon the ability to mount an effective anti-tumor immune response. At a fundamental level, such a response depends on two components. The first is visibility of the tumor, since in order to mount a response the immune system must first recognize the tumor as foreign. The second is T cell functionality, since, in the context of anti-PD-(L)1 treatment, tumors are only effectively eliminated by an antigen specific cytotoxic T cell response that is allowed freedom to operate within the tumor microenvironment (Figure 1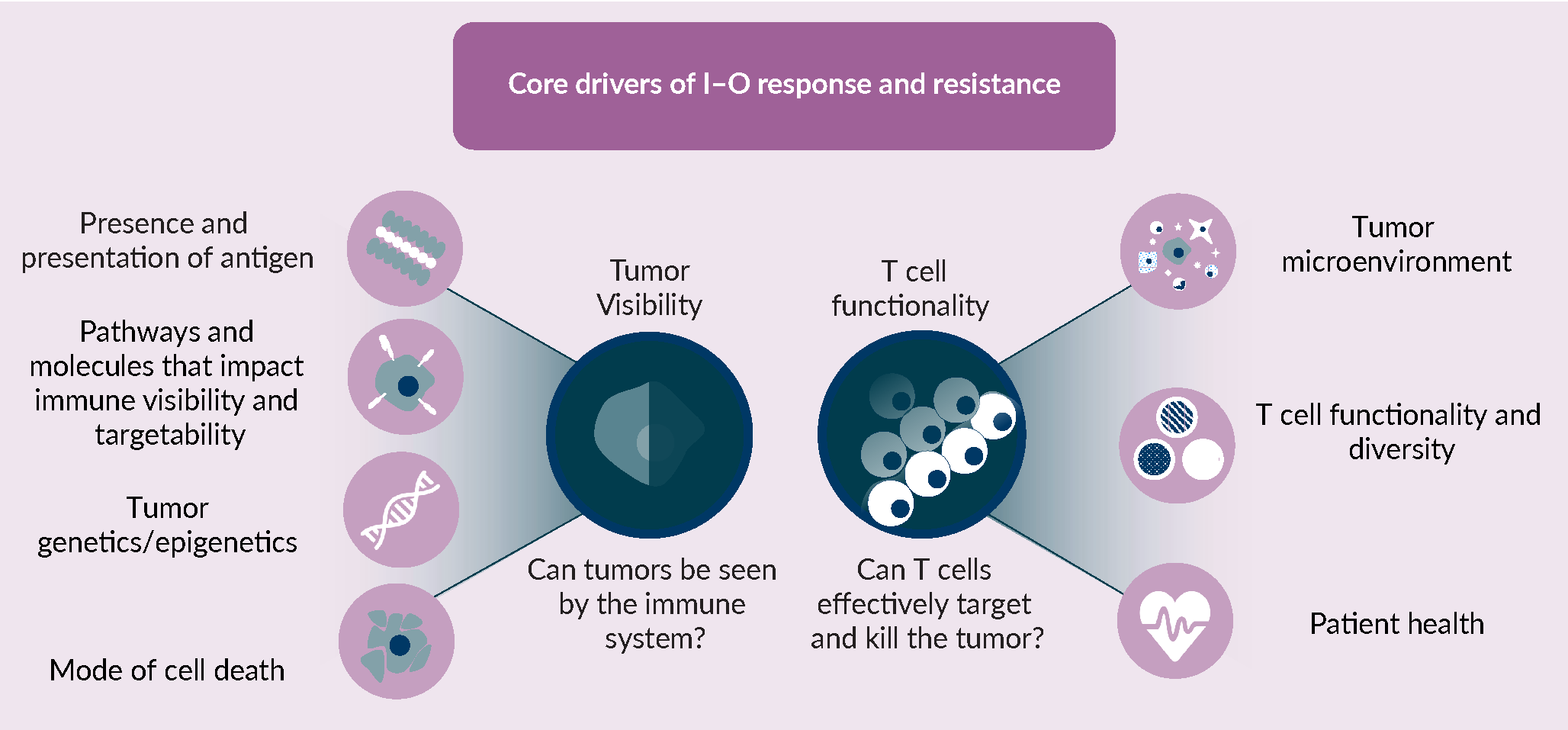 A framework for understanding the core drivers of I–O response and resistance.I–O response and resistance is underpinned by two central drivers: 1) whether or not a tumor is visible to the immune system and; 2) whether or not T cells can effectively target and kill those tumor cells. These two core components, tumor visibility and T cell functionality, are subject to modification by a number of different tumour cell intrinsic and extrinsic aspects of biology, which are themselves interdependent. With respect to tumor visibility, the existence of recognisable neoantigens and their effective presentation via the antigen presentation machinery, pathways and molecules that effect visibility at the tumor cell intrinsic level, such as PD-L1 expression, genetic or epigenetic mutations that can impact both of these features, and the mode of tumor or stromal cell death can all independently or together impact the ability to induce a productive and durable immune response. With respect to T cell functionality, the cells and associated cytokine and chemokine signals within the microenvironment are a major determinant of T cell function within that environment, additionally the overall diversity and functional state of the T cells as well as the patients’ current health status, can have a major role on whether T cells can productively kill tumor cells.). By reducing the significant complexity of the anti-tumor immune response, downstream of anti-PD-(L)1, to these key components, we can formulate and test hypotheses around resistance drivers. Each component can initially be considered in isolation, but eventually together, as part of a network that shapes the two core drivers of tumor visibility and T cell functionality.
A framework for understanding the core drivers of I–O response and resistance.I–O response and resistance is underpinned by two central drivers: 1) whether or not a tumor is visible to the immune system and; 2) whether or not T cells can effectively target and kill those tumor cells. These two core components, tumor visibility and T cell functionality, are subject to modification by a number of different tumour cell intrinsic and extrinsic aspects of biology, which are themselves interdependent. With respect to tumor visibility, the existence of recognisable neoantigens and their effective presentation via the antigen presentation machinery, pathways and molecules that effect visibility at the tumor cell intrinsic level, such as PD-L1 expression, genetic or epigenetic mutations that can impact both of these features, and the mode of tumor or stromal cell death can all independently or together impact the ability to induce a productive and durable immune response. With respect to T cell functionality, the cells and associated cytokine and chemokine signals within the microenvironment are a major determinant of T cell function within that environment, additionally the overall diversity and functional state of the T cells as well as the patients’ current health status, can have a major role on whether T cells can productively kill tumor cells.). By reducing the significant complexity of the anti-tumor immune response, downstream of anti-PD-(L)1, to these key components, we can formulate and test hypotheses around resistance drivers. Each component can initially be considered in isolation, but eventually together, as part of a network that shapes the two core drivers of tumor visibility and T cell functionality.
Presence & presentation of antigens
T cell killing of target cells is dependent upon a T cell receptor (TCR) recognizing a cognate antigen presented in the context of the class I major histocompatibility complex (MHC-I), which is canonically expressed on the surface of all mammalian cells. The majority of antigens presented by tumor cells are ‘self’ antigens, the recognition of which is prevented by the processes of central and peripheral tolerance, in order to avoid widespread tissue damage. However, the mutational processes that underpin oncogenesis have the potential to generate altered-self peptides that are recognizable by T cells; termed ‘neoantigens’.
The presence of such neoantigens is a critical driver of tumor visibility, because in their absence the tumor cell is largely invisible to the T cell repertoire. The relationship between presence of antigen and T cell recognition of tumors is what underpins TMB as a predictive biomarker for response to anti-PD-(L)1. The greater the number of mutations present in a tumor, the higher the probability that a recognizable neoantigen is generated, and that an anti-PD-(L)1 driven T cell response can be stimulated. Neoantigen presence though, like any mutational event, is subject to tumor heterogeneity and clonal evolution in response to treatment. Evidence suggests that tumors with higher levels of clonal neoantigens are more likely to respond to treatment [1]McGranahan N, Furness AJ, Rosenthal R et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Sci. 2016; 351(6280), 1463–1469. and that the loss of neoantigens, in response to treatment, may represent a potential route to acquired resistance [2]Anagnostou V, Smith KN, Forde PM et al. Evolution of Neoantigen Landscape during Immune Checkpoint Blockade in Non-Small Cell Lung Cancer. Cancer Discov. 2017; 7(3), 264–276..
Simply having recognizable antigens is however not enough to guarantee tumor visibility, since recognition is dependent both on the presence of a cognate TCR, which will be touched on later, and also on the presentation of any antigen by MHC at the surface of the cell. The loss of MHC from the surface of tumor cells would represent a significant escape route from T cell mediated cytotoxicity, and mutations in key components of antigen presentation by MHC, such as B2M [3]Sade-Feldman M, Jiao YJ, Chen JH et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 2017; 8(1), 1136. and TAP [4]Rasmussen M, Durhuus JA, Nilbert M, Andersen O, Therkildsen C. Response to Immune Checkpoint Inhibitors Is Affected by Deregulations in the Antigen Presentation Machinery: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022; 12(1). have been associated with resistance to anti-PD-(L)1. Complete loss of MHC is, however, a challenging state for a tumor to maintain, because MHC negative cells are rendered sensitive to killing by natural killer (NK) cells. As an alternative to losing MHC, tumors can also modulate the diversity of MHC present at the genetic or transcriptional level, and by doing so reduce the potential diversity of neoantigens available to the immune system.
Each person carries up to six different MHC I alleles, three inherited from each parent, and increased diversity of these alleles with respect to the peptides they bind, also called the HLA-I evolutionary divergence (HED), has been shown to link to benefit from anti-PD-(L)1 [5]Chowell D, Krishna C, Pierini F et al. Evolutionary divergence of HLA class I genotype impacts efficacy of cancer immunotherapy. Nat. Med. 2019; 25(11), 1715–1720. while loss of heterozygosity (LOH) at the MHC locus has been shown to lead to lack of benefit to anti-PD-(L)1 [6]Montesion M, Murugesan K, Jin DX et al. Somatic HLA Class I Loss Is a Widespread Mechanism of Immune Evasion Which Refines the Use of Tumor Mutational Burden as a Biomarker of Checkpoint Inhibitor Response. Cancer Discov. 2021; 11(2), 282–292.. It is important to note though, that modification of MHC is not only a potential route of immune escape [7]Yu S, Zhao Z, Chen L et al. HLA loss of heterozygosity-mediated discordant responses to immune checkpoint blockade in squamous cell lung cancer with renal metastasis. Immunother. 2021; 13(3), 195–200., but also evidence of selective pressure being applied by an active immune response, and as such may not always associate with reduced benefit from anti-PD-(L)1 [8]Yang Y, Kim E, Kim S. Insignificant effects of loss of heterozygosity in HLA in the efficacy of immune checkpoint blockade treatment. Genes Genomics. 2022; 44(4), 509–515., and in some settings or lines of treatment could actually be a predictor of benefit [9]Litchfield K, Reading JL, Puttick C et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell. 2021; 184(3), 596–614 e514.. A deeper understanding of the role of MHC in resistance and response may be gained by assessing its impact in concert with other markers, such as PD-L1 and CD8, combined with a productive interferon-g (IFN-g) response [10]Kwak Y, Koh J, Park Y et al. Differential prognostic impact of CD8(+) T cells based on human leucocyte antigen I and PD-L1 expression in microsatellite-unstable gastric cancer. Br J Cancer. 2020; 122(9), 1399–1408. [11]Hurkmans DP, Kuipers ME, Smit J et al. Tumor mutational load, CD8(+) T cells, expression of PD-L1 and HLA class I to guide immunotherapy decisions in NSCLC patients. Cancer Immunol. Immunother. 2020; 69(5), 771–777., but will more likely come from advances that improve our ability to measure the expression of individual MHC allotypes, and to link those allotypes to specific neoantigens and cognate TCRs.
Molecules/pathways impacting visibility
The fact that tumor-intrinsic transcriptional programmes play a key role in immune escape and mediating I–O resistance is underlined by several observations. For example, there is wide variability in the response rates to I–O across tumor types with distinct oncogenic signaling processes, and vice versa, the response rates are similar across different histologies when cancers are driven by similar processes such as microsatellite instability. Furthermore, in metastatic disease settings, different tumors from the same individual can have different activity of immunosuppressive pathways, such as Wnt, that track inversely with intra-epithelial infiltration of CD8+ T cells [12]Jimenez-Sanchez A, Memon D, Pourpe S et al. Heterogeneous Tumor-Immune Microenvironments among Differentially Growing Metastases in an Ovarian Cancer Patient. Cell. 2017; 170(5), 927–938 e920. [13]Zhang AW, McPherson A, Milne K, et al. Interfaces of Malignant and Immunologic Clonal Dynamics in Ovarian Cancer. Cell. 2018; 173(7), 1755–1769 e1722. [14]Jimenez-Sanchez A, Cybulska P, Mager KL et al. Unraveling tumor-immune heterogeneity in advanced ovarian cancer uncovers immunogenic effect of chemotherapy. Nat. Genet. 2020; 52(6), 582–593.. This suggests that tumor-intrinsic processes, as well as systemic immune features, can drive the heterogenous tumor-immune microenvironments that are often observed clinically within the same patient. Supporting this, the response to immunotherapy can have clinically diverse temporal and spatial patterns in different sites of the same patient associated with distinct transcriptional programmes, as exemplified in a longitudinal study of an exceptional responder case in a patient will metastatic melanoma [15]Liu D, Lin JR, Robitschek EJ et al. Evolution of delayed resistance to immunotherapy in a melanoma responder. Nat. Med. 2021; 27(6), 985–992.. Thus, a clear understanding of tumor-intrinsic oncogenic programmes and how they shape the anti-tumor immune response in treatment-naïve patients and during treatment with immunotherapy is essential.
Although it remains to be systematically characterized and mechanistically assessed how tumor pathways drive I–O resistance across disease stages and cancer types, emerging evidence from both pre-clinical and clinical insights suggest that a range of cancer pathways can directly or indirectly contribute to modulating the tumor-immune interface. These include Wnt-b-catenin, MAPK, CDK4-6, LKB1 (STK11), PTEN and Myc signaling, the roles for which have been summarized recently [16]Spranger S, Gajewski TF. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat. Rev. Cancer. 2018; 18(3), 139–147. [17]Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 2020; 20(1), 25–39..
While these pathways are associated with I–O resistance in specific settings and indications, a central pathway that is ubiquitously involved in both response and resistance is the IFN-g response pathway; due to its important role in sensing IFN-g secreted from T cells during a productive response against tumor antigen (reviewed in [17]Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 2020; 20(1), 25–39.). IFN-g binds the IFN-g receptor and triggers activation of the Janus kinase (JAK) and signal transducer and activator of transcription (STAT) pathway which in turn activates interferon stimulated genes (ISGs) partially through interferon response factors (IRFs). The ISGs have pleotropic effects with important tumor-intrinsic effects including upregulation of the antigen presentation machinery as well as PD-L1, serving as positive feedback loop enhancing T cell recognition and leading to induction of cell cycle arrest and apoptosis [18]Chin YE, Kitagawa M, Su WC, You ZH, Iwamoto Y, Fu XY. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science. 1996; 272(5262), 719–722.. Tumor-extrinsic effects of ISGs include enhancing the cytolytic activity of both innate and adaptive immune cells. In general, IFN-g sensing by tumor cells leads to a stronger anti-tumorigenic response, particularly in the early stages of tumor development. In line with this, IFN-g response signatures assessed through data analysis from bulk tumors have been associated with better responses to anti-PD1, across cancers [19]Ayers M, Lunceford J, Nebozhyn M, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest. 2017; 127(8), 2930–2940.. Meanwhile alterations of the IFN-g pathway that include JAK-STAT and the antigen presentation machinery have been associated with resistance to I–O (discussed in the following section). Recently, mounting evidence points to the IFN-g response playing a reversed role in mediating pro-tumorigenic effects particularly in the late stages of tumor progression (reviewed in [20]von Locquenghien M, Rozalen C, Celia-Terrassa T. Interferons in cancer immunoediting: sculpting metastasis and immunotherapy response. J. Clin. Invest. 2021; 131(1).). Similarly, pre-clinical evidence in immuno-competent syngeneic B16 mouse models where the cancer cell lines were pre-treated with sustained levels of IFN-g prior to implantation, were found to subsequently develop acquired resistance to ICB in vivo [21]Benci JL, Xu B, Qiu Y et al. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell. 2016; 167(6), 1540–1554 e1512..
Tumor genetics & epigenetics
Historically, a rich source of understanding with respect to response and resistance in oncology, the presence of mutations in one or more genes have yielded less generalizable insights in the context of anti-PD-(L)1 treatment. In NSCLC, it is clear that tumors harboring mutations in dominant oncogenes, such as EGFR and ALK, have limited benefit from anti-PD-(L)1 [22]Lisberg A, Cummings A, Goldman JW et al. A Phase II Study of Pembrolizumab in EGFR-Mutant, PD-L1+, Tyrosine Kinase Inhibitor Naive Patients With Advanced NSCLC. J. Thorac. Oncol. 2018; 13(8), 1138–1145. potentially because these tumors have lower TMB, and so limited opportunity for neoantigen generation, but also because it is challenging to control the growth of such tumors without addressing the central oncogenic drivers of that growth. Mutations in STK11 and KEAP1, while initially proposed as resistance drivers for anti-PD-(L)1, are prognostic in nature [23]Papillon-Cavanagh S, Doshi P, Dobrin R, Szustakowski J, Walsh AM. STK11 and KEAP1 mutations as prognostic biomarkers in an observational real-world lung adenocarcinoma cohort. ESMO Open. 2020; 5(2). [24]Shire NJ, Klein AB, Golozar A et al. STK11 (LKB1) mutations in metastatic NSCLC: Prognostic value in the real world. PLoS One. 2020; 15(9), e0238358., identifying a group of patients that respond poorly to all current therapies. In Melanoma, mutations in the JAK-STAT pathway have been associated with both acquired [25]Zaretsky JM, Garcia-Diaz A, Shin DS et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016; 375(9), 819–829. and primary [26]Shin DS, Zaretsky JM, Escuin-Ordinas H et al. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 2017; 7(2), 188–201. resistance to anti-PD-(L)1, presumably due to the critical nature of these genes with respect to the IFN-g response; itself central to an effective T cell response. Other potential genomic drivers of resistance include PTEN loss and mutations in the WNT/b-Catenin pathway, both of which have been associated with reduced presence of T cells [27]Luke JJ, Bao R, Sweis RF, Spranger S, Gajewski TF. WNT/beta-catenin Pathway Activation Correlates with Immune Exclusion across Human Cancers. Clin. Cancer Res. 2019; 25(10), 3074–3083. [28]Lin Z, Huang L, Li SL, Gu J, Cui X, Zhou Y. PTEN loss correlates with T cell exclusion across human cancers. BMC Cancer. 2021; 21(1), 429. and acquired resistance to anti-PD-(L)1 [29]Trujillo JA, Luke JJ, Zha Y et al. Secondary resistance to immunotherapy associated with beta-catenin pathway activation or PTEN loss in metastatic melanoma. J. Immunother. Cancer. 2019; 7(1), 295.. The majority of these genomic drivers have however not been validated as baseline predictors of response to anti-PD-(L)1 in large, randomized settings, and in a recent meta-analysis, were not seen to be consistent, or statistically significant, with respect to their impact on outcome across a range of studies [9]Litchfield K, Reading JL, Puttick C et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell. 2021; 184(3), 596–614 e514.. More recently deletion of the chromosome 9p21.3 region, containing the genes CDKN2A, CDKN2B and MTAP, has been associated with reduced activity for anti-PD-(L)1 in multiple settings and with reduced immune infiltration [30]Ebot EM, Duncan DL, Tolba K et al. Deletions on 9p21 are associated with worse outcomes after anti-PD-1/PD-L1 monotherapy but not chemoimmunotherapy. NPJ Precis. Oncol. 2022; 6(1), 44.Ebot EM, Duncan DL, Tolba K et al. Deletions on 9p21 are associated with worse outcomes after anti-PD-1/PD-L1 monotherapy but not chemoimmunotherapy. NPJ Precis. Oncol. 2022; 6(1), 44. [31]Han G, Yang G, Hao D et al. 9p21 loss confers a cold tumor immune microenvironment and primary resistance to immune checkpoint therapy. Nat. Commun. 2021; 12(1), 5606., and may represent a promising genomic marker of resistance to anti-PD-(L)1 [30]Ebot EM, Duncan DL, Tolba K et al. Deletions on 9p21 are associated with worse outcomes after anti-PD-1/PD-L1 monotherapy but not chemoimmunotherapy. NPJ Precis. Oncol. 2022; 6(1), 44..
On the opposite side of the spectrum, a number of genomic features have been associated with increased benefit from anti-PD-(L)1, including APOBEC [32]Wang S, Jia M, He Z, Liu XS. APOBEC3B and APOBEC mutational signature as potential predictive markers for immunotherapy response in non-small cell lung cancer. Oncogene. 2018; 37(29), 3924–3936., smoking [33]Yang H, Ma W, Sun B et al. Smoking signature is superior to programmed death-ligand 1 expression in predicting pathological response to neoadjuvant immunotherapy in lung cancer patients. Transl. Lung Cancer Res. 2021; 10(9), 3807–3822. and UV exposure [34]Pham TV, Boichard A, Goodman A et al. Role of ultraviolet mutational signature versus tumor mutation burden in predicting response to immunotherapy. Mol. Oncol. 2020; 14(8), 1680–1694. associated mutational signatures as well as mutations in SERPINB3/4 [35]Riaz N, Havel JJ, Kendall SM et al. Recurrent SERPINB3 and SERPINB4 mutations in patients who respond to anti-CTLA4 immunotherapy. Nat. Genet. 2016; 48(11), 1327–1329., ARID1A [36]Okamura R, Kato S, Lee S, Jimenez RE, Sicklick JK, Kurzrock R. ARID1A alterations function as a biomarker for longer progression-free survival after anti-PD-1/PD-L1 immunotherapy. J. Immunother. Cancer. 2020; 8(1). and KRAS [37]Liu C, Zheng S, Jin R et al. The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer Lett. 2020; 470, 95–105.. Interpreting the independent impact of such genomic features has however been challenged by the fact that many are also associated with increased TMB, a known predictor of improved outcome in many settings, and by the fact that several can have prognostic as well as predictive value in some settings (Figure 2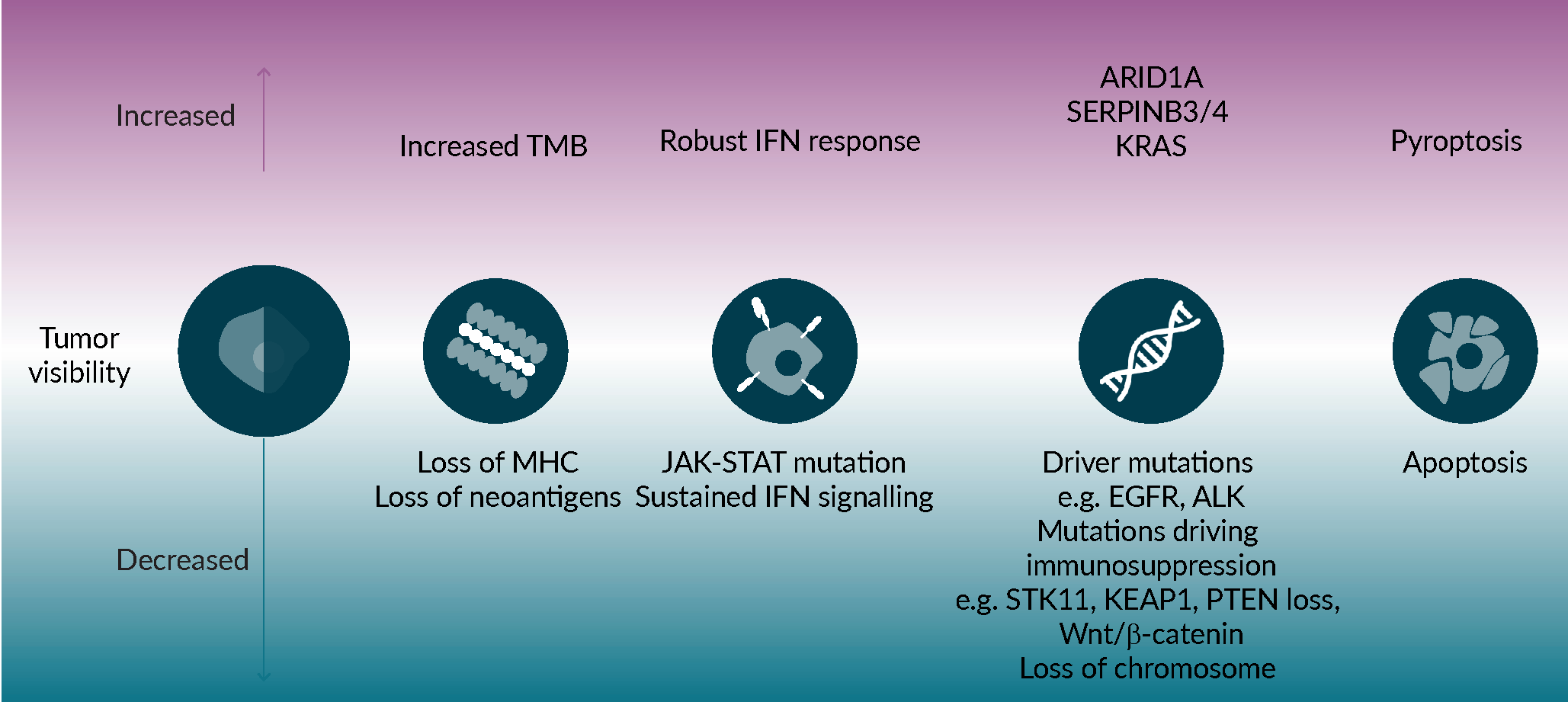 Mechanisms impacting tumor visibility to the immune system.Several tumor-intrinsic mechanisms can affect visibility to immune cells, and therefore susceptibility to immune mediated killing, including decreased tumor mutational burden, inability to present neoantigens or a deregulated antigen presentation machinery, an abnormal IFNγ response, mutations that drive immune suppression and the mode of cell death.).
Mechanisms impacting tumor visibility to the immune system.Several tumor-intrinsic mechanisms can affect visibility to immune cells, and therefore susceptibility to immune mediated killing, including decreased tumor mutational burden, inability to present neoantigens or a deregulated antigen presentation machinery, an abnormal IFNγ response, mutations that drive immune suppression and the mode of cell death.).
While it seems clear that independent, genomic drivers of response and resistance to anti-PD-(L)1 are likely rare, it is inevitable that the genetic background of a tumor will impact the signals it can both receive and produce. Striving to better understand the interface and interlink between tumor genetics and the surrounding inflammatory microenvironment will be critical to deepening our understanding of how tumors and the immune system converse, and to grasping the complexity of response and resistance to anti-PD-(L)1.
Modes of cell death
Death of cancer cells and immune cells occurs spontaneously as well as in response to therapies and pathogens. Multiple modes of regulated cell death occur that may influence the immune response. The major modes of regulated cell death include apoptosis, pyroptosis, ferroptosis, necroptosis, NETosis, autophagic cell death, and cellular senescence (reviewed in [38]Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat. Immunol. 2022; 23(4), 487–500.). Although each process has unique pathways of execution, activation of cysteine proteases of the caspase family is a common theme. Apoptosis activates caspases 3, 8 and 9 whereas caspases 1 and 4 are involved in the proinflammatory process of pyroptosis. Caspases are proposed to connect cell death processes to maintain homeostasis [39]Galluzzi L, Lopez-Soto A, Kumar S, Kroemer G. Caspases Connect Cell-Death Signaling to Organismal Homeostasis. Immunity. 2016; 44(2), 221–231..
From the perspective of response to anti-PD-(L)1, cell death that leads to immunologic memory is key to controlling tumor growth and to therapeutic response. The hallmarks of immunogenic cell death (ICD) are antigenicity, adjuvanticity and environment [38]Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat. Immunol. 2022; 23(4), 487–500.Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat. Immunol. 2022; 23(4), 487–500.Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat. Immunol. 2022; 23(4), 487–500.. Pyroptosis is an evolutionarily conserved mechanism that plays a critical role in innate immune defense to microbial infections. Unlike apoptosis and other cell death processes, pyroptosis results in an inflammatory response. The inflammatory mechanisms driven by viruses, bacteria and some toxicants include the activation of caspase-1 mediated by pattern recognition receptors (PPRs) and a multicomponent complex called the inflammasome. Following pathogen infection, an inflammatory signal is mediated by microbial associated molecular patterns (MAMPs) and toxicants activate damage-associated molecular patterns (DAMP) inflammasomes. MAMPs and DAMPs are sensed by PPRs on myeloid cells that can lead to ICD and a downstream immunologic memory response. A large number of chemotherapies can induce ICD. For example, oxaliplatin but not cis-platinum, can induce ICD in model systems [38]Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat. Immunol. 2022; 23(4), 487–500.Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat. Immunol. 2022; 23(4), 487–500.Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat. Immunol. 2022; 23(4), 487–500.. Caspase-1 activation leads to cleavage of the gasdermin family (GSDMD and GSDME) that oligomerize and form pores in the plasma membrane resulting in release and cleavage of the precursors of the proinflammatory cytokines IL-1b and IL-18. GSDM-D cleavage is mediated by the inflammasome caspases 1, 4,5 and 11 whereas GSDM-E is cleaved by caspases 3 and 8 during apoptosis. This coverts noninflammatory apoptotic signals into pyroptotic death signals, further illustrating the interconnectivity of death processes [40]Zhang Z, Zhang Y, Xia S et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020; 579(7799), 415–420.. GSDM-D is the immediate effector of pyroptosis after inflammatory stimulation, but GSDM-E can enhance IL-1beta release secondarily [41]Zhou B, Abbott DW. Gasdermin E permits interleukin-1 beta release in distinct sublytic and pyroptotic phases. Cell Rep. 2021; 35(2), 108998.. In addition to antigenicity and stimulation of inflammatory adjuvanticity, ICD requires a permissive environment, such as absence of immunosuppressive factors like adenosine, prostaglandin E2 and myeloid derived suppressor cells (MDSCs) [38]Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat. Immunol. 2022; 23(4), 487–500.Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat. Immunol. 2022; 23(4), 487–500.Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat. Immunol. 2022; 23(4), 487–500..
Not all damage inducers or pathogens induce ICD. Features associated with ICD include inhibition of transcription and microtubular disruption, though these mechanisms are yet to be fully understood. A clear distinguishing feature between ICD inducers and cytotoxic agents that do not induce ICD is the activation of the integrated stress response (ISR) pathway which involves phosphorylation of the eukaryotic translation inhibitor factor eIF2a, currently proposed as a pathognomonic biomarker of ICD [38]Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat. Immunol. 2022; 23(4), 487–500.. eIF2a phosphorylation also activates autophagy which can affect cell survival vs death. It is proposed that eIF2a activates a coordinated stress response that protects cells when stress levels are limited and possibly repairable, yet controls cell elimination when damage is extreme [42]Humeau J, Leduc M, Cerrato G, Loos F, Kepp O, Kroemer G. Phosphorylation of eukaryotic initiation factor-2alpha (eIF2alpha) in autophagy. Cell Death Dis. 2020; 11(6), 433.. PDL-1 expression in the nucleus can switch tumor necrosis factor (TNF-a)-induced apoptosis to pyroptosis and tumor necrosis via the transcription of GSDM-C and its cleavage through caspase 8. High levels of GSDM-C correlate with poor prognosis in certain cancers and may represent a non-canonical pathway for pyroptosis and tumor necrosis in cancer cells [43]Hou J, Zhao R, Xia W et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol. 2020; 22(10), 1264–1275.. GSDM-E is often downregulated in cancers with the exception of pancreatic cancers where it has been shown to play a novel function in mediating resistance to digestive enzymes that are produced by pancreatic ducts [44]Lv J, Liu Y, Mo S et al. Gasdermin E mediates resistance of pancreatic adenocarcinoma to enzymatic digestion through a YBX1-mucin pathway. Nat. Cell Biol. 2022; 24(3), 364–372.. The balance between apoptotic and pyroptotic inflammatory cell death is important in cancers. Caspase 3 that mediates apoptotic death, inactivates cGAS and IRF3 that suppress IGFN type 1 production and keep apoptotic death immunologically silent [45]Ning X, Wang Y, Jing M et al. Apoptotic Caspases Suppress Type I Interferon Production via the Cleavage of cGAS, MAVS, and IRF3. Mol Cell. 2019; 74(1), 19–31 e17.. Bcl2 is well known to inhibit apoptosis, but can also reduce GSDM-D activation by dropping caspase 1 cleavage and promoting cleavage at a site D87, a mechanism that inactivates pyroptosis [46]Shi CS, Kehrl JH. Bcl-2 regulates pyroptosis and necroptosis by targeting BH3-like domains in GSDMD and MLKL. Cell Death Discov. 2019; 5, 151.. Targeted therapies can also influence modes of cell death. In BRAF mutated melanoma cells, combination of BRAF and MEK inhibitors can be an effective therapy and induce markers of pyroptosis with concomitant T cell infiltration, a mechanisms that is not present therapy resistant tumors [47]Erkes DA, Cai W, Sanchez IM et al. Mutant BRAF and MEK Inhibitors Regulate the Tumor Immune Microenvironment via Pyroptosis. Cancer Discov. 2020; 10(2), 254–269..
Ferroptosis is another form of non-apoptotic, regulated cell death. It is characterized by iron-dependent accumulation of oxidized polyunsaturated fatty acid containing phospholipids that can lead to membrane rupture and cell death. The pathway was identified by cysteine depletion shown originally to lead to death of cells in culture by Harry Eagle [48]Eagle H. Nutrition needs of mammalian cells in tissue culture. Science. 1955; 122(3168), 501–514., and reviewed in [49]Stockwell BR. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022; 185(14), 2401–2421.. Cysteine levels control the intracellular pool of reduced glutathione (GSH), essential for the activity of the enzyme glutathione peroxidase 4 (GPX4) that reduces peroxidized phospholipids and suppresses activation of the arachidonic acid metabolizing enzymes. Control of ferroptosis mediated death is a complex interplay between lipids, iron and cysteine metabolism. Ferroptosis has been shown to occur in certain cancer cells as well as in immune cells, including T cells, MDSCs, B cells, dendritic cells and NK cells. Ferroptosis susceptibility in cancer cells is influenced by mutations in oncogenes (like TP53 and RAS) and in genes involved in the stress response (NFE2L2), autophagy, hypoxia and epithelial-to-mesenchymal transition (EMT) [50]Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021; 18(5), 280–296.. Different cancers show differing levels of heterogeneity in their susceptibility to ferroptosis which can relate to states of inflammation. CD8 T cells have been found to be able to regulate ferroptosis in tumors during immunotherapy [51]Wang W, Green M, Choi JE et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019; 569(7755), 270–274., through IFN-g, and along with arachidonic acid, can also induce tumor cell ferroptotic death [52]Liao P, Wang W, Wang W et al. CD8(+) T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell. 2022; 40(4), 365–378 e366.. Ferroptosis can also impact immune cells themselves, which can result in immune suppression in certain cancers, for example pathologically activated neutrophils, and PMN-MDSC cells die spontaneously by ferroptosis [53]Kim R, Hashimoto A, Markosyan N et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature. 2022; 612(7939), 338–346..
Through the knowledge we have now gained of the different mechanisms of ICD, and their potential to modulate the tumor microenvironment and tilt the needle to an immune-productive milieu and even sensitize tumors to PD-(L)1 blockade [54]Kepp O, Zitvogel L, Kroemer G. Clinical evidence that immunogenic cell death sensitizes to PD-1/PD-L1 blockade. Oncoimmunol. 2019; 8(10), e1637188., conducting trials where combinations of ICB with either chemotherapy or antibody-drug conjugates (ADCs) are administered using different dosing schedules rather than simultaneously, or even sequentially, may lead to improved patients’ outcomes, with the obvious considerations to take into account, such as toxicity and potential for adverse events.
Tumor microenvironment
It is now well understood that neither tumor progression nor response to IO therapies are solely driven by cancer cell-intrinsic genetic or epigenetic changes, but that these processes are tied to a large communication network of immune cells, stromal tissue, and molecular mediators both within, and at the boundaries of solid tumors. This ecosystem, or tumor microenvironment (TME), through its dynamic and multi-directional interactions with the tumor and the immune system can be both friend and foe with respect to response to anti-PD-(L)1.
Several studies have shown that a microenvironment with a coordinated, Th1 immune response is far more likely to respond to anti-PD-(L)1 therapy. Such an environment is characterized by increased CD8 infiltration [55]Tumeh PC, Harview CL, Yearley JH et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014; 515(7528), 568–571., evidence of active IFN-g signaling [19]Ayers M, Lunceford J, Nebozhyn M, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest. 2017; 127(8), 2930–2940. and presence of tertiary lymphoid structures [56]Vanhersecke L, Brunet M, Guegan JP et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat. Cancer. 2021; 2(8), 794–802.. Through direct interactions and release of signaling molecules, cancer cells can co-opt the microenvironment, creating an immune-suppressive milieu, in which, the stromal compartment cooperates to promote tumor growth and metastases, and immune cells are modulated to a suppressive state. Evasion of the immune system and chronic inflammation, which can generate a forward feeding circle, are well recognized hallmarks of cancer [57]Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022; 12(1), 31–46., and have the potential to create a significant barrier to the activity of anti-PD-(L)1 [58]Denton AE, Roberts EW, Fearon DT. Stromal Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2018; 1060, 99–114.. In this section, we focus on the immune cell components of the TME, and their potential to positively and negatively impact such activity. Although we recognize there are some reports of stromal cells other than immune cells playing a role in resistance to I–O therapy [59]Kim BG, Malek E, Choi SH, Ignatz-Hoover JJ, Driscoll JJ. Novel therapies emerging in oncology to target the TGF-beta pathway. J. Hematol. Oncol. 2021; 14(1), 55.Kim BG, Malek E, Choi SH, Ignatz-Hoover JJ, Driscoll JJ. Novel therapies emerging in oncology to target the TGF-beta pathway. J. Hematol. Oncol. 2021; 14(1), 55., and strategies that target the stromal compartment are beginning to emerge [59]Kim BG, Malek E, Choi SH, Ignatz-Hoover JJ, Driscoll JJ. Novel therapies emerging in oncology to target the TGF-beta pathway. J. Hematol. Oncol. 2021; 14(1), 55.Kim BG, Malek E, Choi SH, Ignatz-Hoover JJ, Driscoll JJ. Novel therapies emerging in oncology to target the TGF-beta pathway. J. Hematol. Oncol. 2021; 14(1), 55., we do not consider them in depth here.
Innate immune cells in the TME
Tissue resident and circulating innate immune cells are key contributors to the inflammatory state of the TME, with major players having been identified in both the myeloid and lymphoid lineages. Innate immune cells, like macrophages, neutrophils, NK cells and innate lymphoid cells (ILCs) provide a bridge to adaptive immunity, and their interactions with T cells through either soluble mediators and/or cell-cell interactions warrant some exploration to understand the mechanisms by which they can impact resistance to I–O therapy. Tumor associated macrophages (TAMs) are one of the most abundant immune cell populations in the TME, and their density in the tumor tissue has been correlated to poor outcomes and resistance to immunotherapy [60]Jung KY, Cho SW, Kim YA et al. Cancers with Higher Density of Tumor-Associated Macrophages Were Associated with Poor Survival Rates. J. Pathol. Transl. Med. 2015; 49(4), 318–324.. Generally, macrophages in the TME are thought to exhibit one of two phenotypes, a classically activated or pro-inflammatory, antigen presenting, M1 phenotype, or the alternatively activated, anti-inflammatory, M2 phenotype, each defined by expression of different surface markers, and cytokine secretome. However, in reality, a degree of plasticity has been observed in TAM phenotypes and functions, depending on the tumor milieu, the stage of development of the tumor, and the cancer type [61]Christofides A, Strauss L, Yeo A, Cao C, Charest A, Boussiotis VA. The complex role of tumor-infiltrating macrophages. Nat. Immunol. 2022; 23(8), 1148–1156.. They have been demonstrated, both pre-clinically and in human tumors, to contribute to a pro-tumorigenic TME through promotion of angiogenesis and tumor metastases [62]Duan Z, Luo Y. Targeting macrophages in cancer immunotherapy. Signal Transduct. Target Ther. 2021; 6(1), 127., and while their biology within the TME is complex, some trends are emerging that suggest that they likely play a role in resistance to chemotherapy, radiotherapy and ICB [63]Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015; 27(4), 462–472.. As such combination therapies that aim at targeting macrophages, together with anti-PD-(L)1 may help with overcoming resistance. Multiple potential mechanisms of macrophage modulation could be considered including modifying survival or recruitment in or order to reduce their presence in the TME, re-polarizing M2 to M1 and re-educating TAMs to an anti-tumor, pro-inflammatory function, or through blockade of myeloid immune checkpoints that induce a pro-phagocytic phenotype [64]Yu J, Green MD, Li S et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat. Med. 2021; 27(1), 152–164..
The number of phase 1 and 2 clinical trials that include myeloid targeting agents in combination with I–O is starting to take off, shifting the landscape away from single I–O agents. The challenges with therapies that target myeloid cells, like macrophages and other myeloid-derived heterogenous cell types with a known T cell inhibition/ Treg inducing role in TME and impact on ICB resistance such as myeloid-derived suppressive cells (MDSCs) [65]Li T, Liu T, Zhu W et al. Targeting MDSC for Immune-Checkpoint Blockade in Cancer Immunotherapy: Current Progress and New Prospects. Clin. Med. Insights Oncol. 2021; 15, 11795549211035540., will be to dissect the mechanisms by which they modulate resistance to I–O in different indications, and throughout tumor development, and to effectively address combinations in different tumor types with evidence-based dosing schedules.
Dendritic cells (DCs) represent another innate, tissue resident cell with a major role in the antitumor immune response. They are the professional antigen presenting cells (APCs) that detect environmental signals – tumor associated and tumor specific antigens (TAAs and TSAs) – and shape T cell mediated immunity, by transporting these antigens from tumor to lymph nodes, and inducing robust CD8 T cell priming, a process that has been shown to be dependent on type I IFN signaling [66]Gardner A, de Mingo Pulido A, Ruffell B. Dendritic Cells and Their Role in Immunotherapy. Front. Immunol. 2020; 11, 924. presence of conventional DCs in tumors correlated with improved response to PD-1 therapy and higher CD8 T cell infiltration and was generally associated with better prognosis in several indications [67]Busa R, Bulati M, Badami E et al. Tissue-Resident Innate Immune Cell-Based Therapy: A Cornerstone of Immunotherapy Strategies for Cancer Treatment. Front. Cell Dev. Biol. 2022; 10, 907572.. Lack of tumor immunogenicity has been identified as a key factor contributing to anti-PD-(L)1 resistance, and effective DC-T cell crosstalk through the IFN-g and IL-12 axis has been shown pre-clinically to be a critical requirement for both priming and function of cytotoxic T cells, and for the success of anti-PD-(L)1 therapy [68]Garris CS, Arlauckas SP, Kohler RH et al. Successful Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-gamma and IL-12. Immunity. 2022; 55(9), 1749.. So far, there has been surprisingly little clinical evidence of effectiveness for therapies aimed at enhancing DC function in different indications, such as DC vaccine approaches using ex vivo generated autologous DCs from blood-derived monocytes pulsed with TAAs, with only one FDA approval for DC cell therapy, based on a 4.1 month survival improvement and delay in disease progression in metastatic prostate cancer [69]Kantoff PW, Higano CS, Shore ND et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010; 363(5), 411–422.. This is likely due to the immunosuppressive environment of the TME, and thus future combinatorial strategies may focus on rendering the TME more favorable to effector cell infiltration and reveal a potential for TAA-loaded DC cell vaccines. There are challenges to DC targeting, however, including the lack of biomarkers for patient stratification, the cost of personalized therapy, the influence of the immune suppressive TME and lack of mechanistic understanding of how DCs can help overcome resistance to ICB through T cell mediated immunity, as thus far most of knowledge of DC biology comes primarily from animal models.
On the lymphoid arm of innate immunity, NK cells are well recognized as the frontline innate cytotoxic T cell counterparts for tumor cell killing. NK cells are tightly regulated through a balance of activating or inhibitory signals that are highly dependent on cellular and molecular cues from the microenvironment within the tissue where they circulate. In homeostasis, recognition of MHC-I by NK killer Ig-like inhibitory receptors (KIRs) and NKG2A provides a signal that induces self-tolerance and the distinction between healthy ‘self’ or tumor or virus-infected ‘missing-self’ [70]Islam R, Pupovac A, Evtimov V et al. Enhancing a Natural Killer: Modification of NK Cells for Cancer Immunotherapy. Cells. 2021; 10(5).. One of the known tumor evasion mechanisms is the downregulation of MHC-I to escape from CD8 T cell-mediated killing, an event that should activate NK cells to control tumor progression. However, other co-signals are necessary for a full activation cascade and a concomitant productive cytotoxic response by the NK cells, and these pathways are highly dysregulated within the tumor tissue, reducing NK cell
effectiveness [71]Wong JKM, Dolcetti R, Rhee H, Simpson F, Souza-Fonseca-Guimaraes F. Weaponizing natural killer cells for solid cancer immunotherapy. Trends Cancer. 2022..
Increased numbers and fitness of NK cells in the tumor tissue have been associated with better survival outcomes in several different types of cancers [72]Chiossone L, Vivier E. Bringing natural killer cells to the clinic. J. Exp. Med. 2022; 219(10).. However, in NSCLC, elevated NK cells have been associated with worse prognosis, and these cells were found to express high levels of inhibitory receptors, and present an immature, and pro-angiogenic phenotype [73]Bruno A, Focaccetti C, Pagani A et al. The proangiogenic phenotype of natural killer cells in patients with non-small cell lung cancer. Neoplasia. 2013; 15(2):, 133–142.. Despite seemingly conflicting reports, the fact that NK cells harbor surface expression of CTLA-4, PD-1, TIM-3 and TIGIT, are equipped with Fc-mediated effector functions that can be triggered by therapeutic monoclonal antibodies (mAbs), and secrete a myriad of cytokines such as IFN-g, TNF-a, amongst others, that contribute to adaptive immune cell infiltration and activation ([70]Islam R, Pupovac A, Evtimov V et al. Enhancing a Natural Killer: Modification of NK Cells for Cancer Immunotherapy. Cells. 2021; 10(5)., means that leveraging NK cell biology to augment response to anti-PD-(L)1 may become a successful therapeutic strategy. Indeed promising results have already been observed for the combination of pembrolizumab and allogeneic NK cells [74]Lin M, Luo H, Liang S et al. Pembrolizumab plus allogeneic NK cells in advanced non-small cell lung cancer patients. J. Clin. Invest. 2020; 130(5), 2560–2569. and for durvalumab and, the NKG2A targeting antibody, Monalizumab in NSCLC [75]Herbst RS, Majem M, Barlesi F et al. COAST: An Open-Label, Phase II, Multidrug Platform Study of Durvalumab Alone or in Combination With Oleclumab or Monalizumab in Patients With Unresectable, Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022; 40(29), 3383–3393..
Neutrophils typically account for 70% of total white blood cells in peripheral blood, playing a crucial role as a first responder in infection [76]Ley K, Hoffman HM, Kubes P et al. Neutrophils: New insights and open questions. Sci. Immunol. 2018; 3(30).. However, their role of in cancer has been debatable until now, with both pro- and anti-tumor properties having been assigned to this cell type, likely due to the fact that they can retain some functional plasticity and can respond differently to cues in their microenvironment [77]Sionov RV, Fridlender ZG, Granot Z. The Multifaceted Roles Neutrophils Play in the Tumor Microenvironment. Cancer Microenviron. 2015; 8(3), 125–158.. Three populations of neutrophils have been identified in the circulation in cancer patients, mature high density neutrophils (HDN), mature low density neutrophils (LDN) and immature LDNs, with associated cytotoxic phenotypes for the first and immune suppressive for the latter two [78]Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 2019; 16(10), 601–620.. In a retrospective data analysis of single cell RNA-seq from 29 public datasets in NSCLC, a tumor resident neutrophil signature was found to be associated with atezolizumab treatment failure, and identified as a potential negative prognostic biomarker [79]Salcher S, Sturm G, Horvath L et al. High-resolution single-cell atlas reveals diversity and plasticity of tissue-resident neutrophils in non-small cell lung cancer. Cancer Cell. 2022.. In line with these findings, a prospective study monitoring circulating LDNs in NSCLC has similarly found that elevated baseline LDNs could predict primary resistance to first line anti-PD-(L)1 therapy. The authors further their investigation and proposed that the mechanisms by which neutrophils might confer resistance are through soluble molecules within the HGF/c-MET pathway which were in an ex vivo setting able to dampen T cell cytotoxicity. Interestingly, in the same study, the cohort receiving ICB therapy in combination with chemotherapy, there was no association between high levels of LDN and resistance to treatment and authors suggest that this combination may favorably deplete neutrophils and hence potentiate the effect of immunotherapy [80]Arasanz H, Bocanegra AI, Morilla I et al. Circulating Low Density Neutrophils Are Associated with Resistance to First Line Anti-PD1/PDL1 Immunotherapy in Non-Small Cell Lung Cancer. Cancers (Basel). 2022; 14(16).. In contrast to these findings in NSCLC, the neutrophil-to-lymphocyte ratio (NLR) in head and neck and salivary cancers was found to correlate with survival outcomes but not with response to pembrolizumab and vorinostat [81]Pan C, Wu QV, Voutsinas J et al. Neutrophil to lymphocyte ratio and peripheral blood biomarkers correlate with survival outcomes but not response among head and neck and salivary cancer treated with pembrolizumab and vorinostat. Head Neck. 2022., highlighting a need to better define the functional role of neutrophils in the TME. Additionally, it is critical to define what is a true prognostic or predictive measure for this biomarker that can be standardized to be used across tumor indications, in order to truly understand its value and differences across cancer types for better patient stratification.
immune cells in the TME
Given their critical nature with respect to the anti-tumor immune response, it is perhaps of no surprise that increased infiltration of tumors by CD8 T cells has been associated with both improved prognosis [82]Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010; 29(8), 1093–1102. and improved response to anti-PD-(L)1 [83]Li F, Li C, Cai X et al. The association between CD8+ tumor-infiltrating lymphocytes and the clinical outcome of cancer immunotherapy: A systematic review and meta-analysis. EClinicalMedicine. 2021; 41, 101134. in a number of settings and studies. Despite this, patients with comparable levels of CD8 infiltrate can have disparate responses to treatment, this could be driven by differences in the accessibility of some tumoral regions [84]Hammerl D, Martens JWM, Timmermans M et al. Spatial immunophenotypes predict response to anti-PD1 treatment and capture distinct paths of T cell evasion in triple negative breast cancer. Nat. Commun. 2021; 12(1), 5668., but also due to the fact that a measurable proportion of infiltrating CD8s are bystanders, with specificity for common pathogens such as EBV and CMV [85]Simoni Y, Becht E, Fehlings M et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018; 557(7706), 575–579.. Recent studies have begun to try and unpick some of the complexity of CD8 populations within the tumor, utilizing techniques such as single cell Ranse to define signatures of neoantigen specific CD8s [86]Lowery FJ, Krishna S, Yossef R et al. Molecular signatures of antitumor neoantigen-reactive T cells from metastatic human cancers. Science. 2022; 375(6583), 877–884. and using flow cytometry to identify a stem like population, characterized by TCF1 expression [87]Jansen CS, Prokhnevska N, Master VA et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature. 2019; 576(7787), 465–470.. This stem-like sub-set gave rise to more terminally differentiated effectors, and was resident within niches populated by antigen presenting cells, the lack of which was associated with more rapid progression in renal cancer [88]Gao J, Ward JF, Pettaway CA et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat. Med. 2017; 23(5), 551–555..
In classical immunology, CD4 T cells are a central player, given their critical role providing support for both B cell and CD8 T cell activation [89]Tay RE, Richardson EK, Toh HC. Revisiting the role of CD4(+) T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene Ther. 2021; 28(1–2), 5–17.. Their role in the anti-tumor immune response, and particularly in the response to anti-PD-(L)1, is far less well defined. Recently a CXCL13+ population of highly clonal intratumoral CD4 T cells has been identified in a range of tumor types [90]Cohen M, Giladi A, Barboy O et al. The interaction of CD4(+) helper T cells with dendritic cells shapes the tumor microenvironment and immune checkpoint blockade response. Nat. Cancer. 2022; 3(3), 303–317.. This population was shown to associate with antigen presenting cells within the TME, and has been associated with response to anti-PD-(L)1 in breast cancer [91]Zhang Y, Chen H, Mo H et al. Single-cell analyses reveal key immune cell subsets associated with response to PD-L1 blockade in triple-negative breast cancer. Cancer Cell. 2021; 39(12), 1578–1593 e1578.. CXCL13 is a key chemokine involved in the recruitment and organization of B cells within the lymphoid follicles [92]Kazanietz MG, Durando M, Cooke M. CXCL13 and Its Receptor CXCR5 in Cancer: Inflammation, Immune Response, and Beyond. Front Endocrinol (Lausanne). 2019; 10, 471., which itself has been associated with improved response to anti-PD-(L)1 in bladder cancer [93]Goswami S, Chen Y, Anandhan S et al. ARID1A mutation plus CXCL13 expression act as combinatorial biomarkers to predict responses to immune checkpoint therapy in mUCC. Sci. Transl. Med. 2020; 12(548)., and it is tempting to posit that the role of these CD4, CXCL13+ cells in the context of the tumor, may be to help drive assembly of TLS like structures that mimic these follicles to some degree, promoting antigen presentation and activation of downstream CD8 T cells.
As part of their inherent plasticity, CD4 T cells are known to come in a range of ‘flavors’, e.g. TH1, TH2, TH17, etc., each aligned largely to the cytokines they predominantly produce and each with differing primary functions. One CD4 sub-set of particular relevance to resistance is regulatory T cells, Tregs, typically defined by the expression of the transcription factor FOXP3. These cells have been proposed to play a suppressive role, inhibiting the activity of CD8 T cells, and have been associated with poor prognosis in a range of cancer types [94]Saleh R, Elkord E. FoxP3(+) T regulatory cells in cancer: Prognostic biomarkers and therapeutic targets. Cancer Lett. 2020; 490, 174–185.. Interestingly there are very few studies in which the expression of FOXP3 has been explored with respect to anti-PD-(L)1 response, and in one such study in bladder cancer the presence of FOXP3+ cells was actually associated with improved responses [95]Szabados B, Kockx M, Assaf ZJ et al. Final Results of Neoadjuvant Atezolizumab in Cisplatin-ineligible Patients with Muscle-invasive Urothelial Cancer of the Bladder. Eur. Urol. 2022; 82(2), 212–222.. One confounding factor, mentioned by the authors, is that Treg infiltration tends to correlate to CD8 T cell infiltration, and so assessing FOXP3 as an independent marker is a challenge. It is possible that additional studies in other settings may reveal a more critical role for Tregs in resistance to anti-PD-(L)1, but to date clinical evidence of this has not been forthcoming.
The other critical component of the adaptive immune response, the B cell, has for many years been largely unexplored with respect to its impact on response to anti-PD-(L)1 therapy. This lack of attention may have stemmed from the mixed results observed with respect to the prognostic impact of B cells in cancer [96]Wouters MCA, Nelson BH. Prognostic Significance of Tumor-Infiltrating B Cells and Plasma Cells in Human Cancer. Clin. Cancer Res. 2018; 24(24), 6125–6135., and the lack of clear evidence that the humoral response was key to tumor rejection. However, the other critical role for B cells, outside of antibody production, is driving antigen presentation and priming of the cellular response. More recently, a number of studies have highlighted the potential importance of this aspect of B cell biology with respect to anti-PD-(L)1 therapy. Studies in both melanoma [97]Cabrita R, Lauss M, Sanna A et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020; 577(7791), 561–565.Cabrita R, Lauss M, Sanna A et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020; 577(7791), 561–565. [98]Helmink BA, Reddy SM, Gao J et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020; 577(7791), 549–555. [99]Griss J, Bauer W, Wagner C et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat. Commun. 2019; 10(1), 4186. NSCLC [100]Patil NS, Nabet BY, Muller S et al. Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung cancer. Cancer Cell. 2022; 40(3), 289–300 e284. have illustrated the relationship between the presence of B cells, and the formation of TLS, and improved benefit following anti-PD-(L)1 treatment. In one of these studies [97]Cabrita R, Lauss M, Sanna A et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020; 577(7791), 561–565., it was the patients with both TLS and high levels of CD8 infiltrate that gleaned the highest benefit.
These data, together with those discussed above for innate immune cells, suggest that it is a coordinated and holistic response, in which all parts of the immune system are engaged and directionally aligned, that primes for response to anti-PD-(L)1 (Figure 3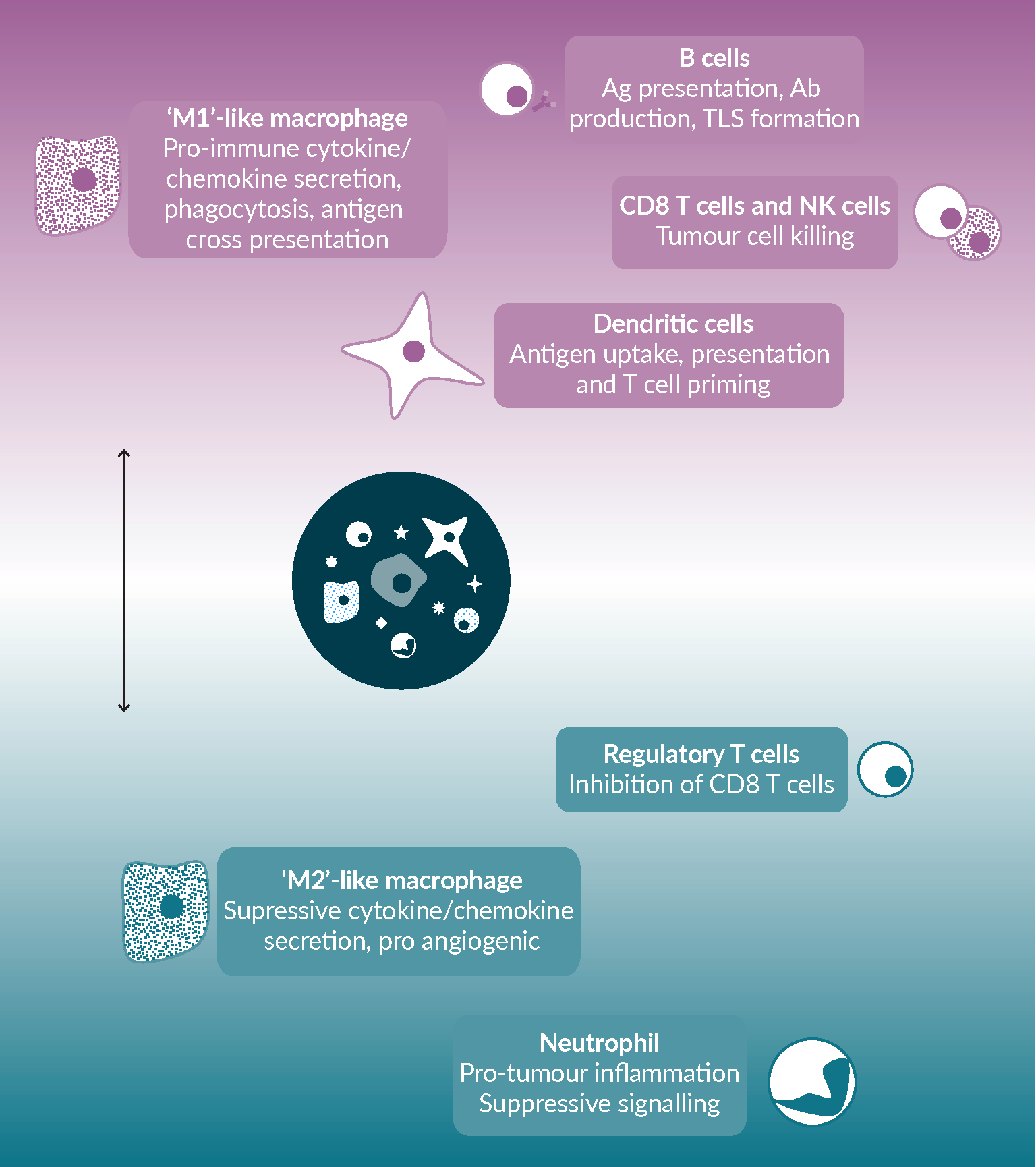 The tumor microenvironment has a major role in shaping the T cell response, and the potential effectiveness of anti-PD-(L)1.Multiple immune cell types populate the tumor microenvironment, and while most of these cell types can have diverse impact on the anti-tumor immune response, they can also be broadly classified into those, highlighted in green, that promote anti-tumor immunity and which therapeutic approaches would aim to enhance, and those, highlighted in red, that promote immunosuppression and which therapeutic approaches would aim to remove or reprogram.). The key questions that now present themselves are what are the factors that prevent that holistic response, are they common across patients or unique in every case and are there means by which we can intervene to overcome them and promote a more effective immune response. Delivering answers to these questions will be facilitated by advances in our ability to study and characterize the TME, such as single cell RNAseq, mass cytometry and multi-parameter immunofluorescence. It is only with these approaches that we can begin to dissect the many interactions and moving parts within the TME and understand how best to modify them.
The tumor microenvironment has a major role in shaping the T cell response, and the potential effectiveness of anti-PD-(L)1.Multiple immune cell types populate the tumor microenvironment, and while most of these cell types can have diverse impact on the anti-tumor immune response, they can also be broadly classified into those, highlighted in green, that promote anti-tumor immunity and which therapeutic approaches would aim to enhance, and those, highlighted in red, that promote immunosuppression and which therapeutic approaches would aim to remove or reprogram.). The key questions that now present themselves are what are the factors that prevent that holistic response, are they common across patients or unique in every case and are there means by which we can intervene to overcome them and promote a more effective immune response. Delivering answers to these questions will be facilitated by advances in our ability to study and characterize the TME, such as single cell RNAseq, mass cytometry and multi-parameter immunofluorescence. It is only with these approaches that we can begin to dissect the many interactions and moving parts within the TME and understand how best to modify them.
T cell functionality & diversity
As described above, while increased CD8 infiltration into tumors has been shown to predict anti-PD-(L)1 activity, not all CD8 cells in the tumor will be relevant to the anti-tumor response. One way to better understand the nature of T cell populations is through profiling of the T cell receptor (TCR) repertoire. Such sequencing allows an assessment of the composition of the repertoire with respect to its diversity, i.e. how many different TCRs are present, and its evenness or clonality, i.e. does each TCR make up a comparable proportion of the population or is the population dominated by a small number of clones. Treatment with anti-PD-(L)1 therapy has been shown to alter the TCR repertoire within the tumor, leading to increased clonality [55]Tumeh PC, Harview CL, Yearley JH et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014; 515(7528), 568–571. [101]Riaz N, Havel JJ, Makarov V et al. Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell. 2017; 171(4), 934–949 e916.Riaz N, Havel JJ, Makarov V et al. Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell. 2017; 171(4), 934–949 e916.. These changes in clonality were accompanied by loss of neoantigens and changes in neoantigen clonality [101]Riaz N, Havel JJ, Makarov V et al. Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell. 2017; 171(4), 934–949 e916., supporting the concept that expansion of tumor-specific T cells is driving this increased clonality. Interestingly, increased intratumoral clonality prior to treatment has been associated with improved response to anti-PD-(L)1, while increased diversity has been associated with improved response to non-PD-(L)1 based therapy [102]Valpione S, Mundra PA, Galvani E et al. The T cell receptor repertoire of tumor infiltrating T cells is predictive and prognostic for cancer survival. Nat. Commun. 2021; 12(1), 4098..
Increases in clonality, similar to those described for intratumoral T cells, can also be observed in peripheral blood following treatment with anti-PD-(L)1 [103]Kato T, Kiyotani K, Tomiyama E et al. Peripheral T cell receptor repertoire features predict durable responses to anti-PD-1 inhibitor monotherapy in advanced renal cell carcinoma. Oncoimmunol. 2021; 10(1), 1862948.. Interestingly treatment with anti-CTLA-4 based therapy appears to have the opposite impact on the TCR repertoire, driving increased diversity in a number of studies [104]Cha E, Klinger M, Hou Y, et al. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci. Transl. Med. 2014; 6(238), 238ra270. [105]Kvistborg P, Philips D, Kelderman S et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci. Transl. Med. 2014; 6(254), 254ra128.. In one study, exploring sequential treatment, clonality was not associated with outcome on anti-CTLA-4, but patients with increased clonality following CTLA-4 demonstrated increased benefit from subsequent anti-PD-(L)1. These data are in keeping with the concept that anti-PD-(L)1 functions, at least in part, to enhance activation of existing anti-tumor T cells while anti-CTLA-4 predominantly acts to drive activation of T cells not already participating in the anti-tumor immune response.
One potential advantage of TCR repertoire analyses is that they can be conducted on a blood sample, potentially allowing for less invasive biomarker monitoring. However, there have been very mixed results to date with respect to the predictive value of such peripheral measures [106]Kidman J, Principe N, Watson M et al. Characteristics of TCR Repertoire Associated With Successful Immune Checkpoint Therapy Responses. Front. Immunol. 2020; 11, 587014.. This is perhaps unsurprising since only a fraction of the peripheral repertoire consists of anti-tumor T cells. In a recent study, increased diversity in flow cytometrically isolated PD-1+, CD8+ T cells from the peripheral blood was associated with improved response to anti-PD-(L)1 [107]Han J, Duan J, Bai H et al. TCR Repertoire Diversity of Peripheral PD-1(+)CD8(+) T Cells Predicts Clinical Outcomes after Immunotherapy in Patients with Non-Small Cell Lung Cancer. Cancer Immunol. Res. 2020; 8(1), 146–154.. This suggests that one potential approach to improving the utility of peripheral measures could be to focus analysis on cells more likely to be tumor-reactive.
While the TCR sequence of a T cell defines its specificity, it does not say anything about its functional state, which is also a potentially important driver of response to anti-PD-(L)1. The concept of T cell exhaustion, through chronic stimulation, is one that has underpinned the development of PD-(L)1 targeting agents. Measuring exhaustion functionally in a clinical context remains very challenging, but attempts have been made to measure it phenotypically via expression of a number of surface receptors such as PD-1, TIM-3 and LAG-3 [108]Jiang W, He Y, He W et al. Exhausted CD8+T Cells in the Tumor Immune Microenvironment: New Pathways to Therapy. Front. Immunol. 2020; 11, 622509.. Expression of these markers has been associated with reduced activity for anti-PD-(L)1 in some studies [109]Lopez de Rodas M, Nagineni V, Ravi A et al. Role of tumor infiltrating lymphocytes and spatial immune heterogeneity in sensitivity to PD-1 axis blockers in non-small cell lung cancer. J. Immunother. Cancer. 2022; 10(6). [110]Datar I, Sanmamed MF, Wang J et al. Expression Analysis and Significance of PD-1, LAG-3, and TIM-3 in Human Non-Small Cell Lung Cancer Using Spatially Resolved and Multiparametric Single-Cell Analysis. Clin. Cancer Res. 2019; 25(15), 4663–4673., but the extent of impact and the role of one marker vs the other are not necessarily consistent. This lack of consistency may be driven by the fact that many exhaustion markers are also activation markers, which correlate to each other and other markers associated with response such as CD8 infiltration and PD-L1 expression. These complex relationships confound analysis relative to outcome and almost demand a move to technologies that allow analysis at the single cell level.
Patient health
A number of susceptibility factors related to patient heath influences the initial response to anti-PD-(L)1. These include high levels of inflammatory markers such as C reactive protein (CRP) or lactate dehydrogenase (LDH) [111]Awada G, Jansen Y, Schwarze JK et al. A Comprehensive Analysis of Baseline Clinical Characteristics and Biomarkers Associated with Outcome in Advanced Melanoma Patients Treated with Pembrolizumab. Cancers (Basel). 2021; 13(2). [112]Minichsdorfer C, Gleiss A, Aretin MB, Schmidinger M, Fuereder T. Serum parameters as prognostic biomarkers in a real world cancer patient population treated with anti PD-1/PD-L1 therapy. Ann Med. 2022; 54(1), 1339–1349., reduced body weight or cachexia [113]Rounis K, Makrakis D, Tsigkas AP et al. Cancer cachexia syndrome and clinical outcome in patients with metastatic non-small cell lung cancer treated with PD-1/PD-L1 inhibitors: results from a prospective, observational study. Transl. Lung Cancer Res. 2021; 10(8), 3538–3549. [114]Cortellini A, Bersanelli M, Buti S et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J. Immunother. Cancer. 2019; 7(1), 57., presence of liver metastasis [115]Xie M, Li N, Xu X et al. The Efficacy of PD-1/PD-L1 Inhibitors in Patients with Liver Metastasis of Non-Small Cell Lung Cancer: A Real-World Study. Cancers (Basel). 2022; 14(17). [116]Wang X, Ji Q, Yan X et al. The Impact of Liver Metastasis on Anti-PD-1 Monoclonal Antibody Monotherapy in Advanced Melanoma: Analysis of Five Clinical Studies. Front. Oncol. 2020; 10, 546604. and age [117]Nosrati A, Tsai KK, Goldinger SM et al. Evaluation of clinicopathological factors in PD-1 response: derivation and validation of a prediction scale for response to PD-1 monotherapy. Br. J. Cancer. 2017; 116(9), 1141–1147.. Many of these factors are prognostic in nature, and impact response to all cancer treatments, but given their close relationship to inflammation and hematological health, they may have a uniquely significant contribution with respect to response to immunotherapies such as anti-PD-(L)1. Large, randomized data sets combined with the power of machine learning approaches may enable us to begin to pull apart which of these factors are most important and in which settings.
Translational Insight
Here we proposed a framework to understand I–O resistance based on two fundamental questions: is the tumor visible to the immune system and are the T cells fit enough to kill the tumor? Each of these two aspects is subject to modification by a number of interlinked tumor cell intrinsic and extrinsic aspects of biology. By reducing the significant complexity of the anti-tumor immune response, downstream of anti-PD-(L)1, to these key components, we can formulate and test hypotheses around resistance drivers and appropriate combinations to overcome them. For example, in patients with negligible neoantigen burden, or total loss of antigen presentation machinery alternatives to anti-PD-(L)1 may be more appropriate, such as targeted T cell engagers or antibody drug conjugates. In patients where T cell exhaustion appears to be restraining effective responses, overcoming this exhaustion through blockade of additional checkpoint receptors, such as TIM-3, or through blockade of downstream signaling molecules such as HPK1 could provide a path forward. In tumors with molecular drivers of resistance, those drivers may bring with them alternative targetable susceptibilities, for example loss of chromosome 9 results in loss of MTAP, [118]Kryukov GV, Wilson FH, Ruth JR et al. MTAP deletion confers enhanced dependency on the PRMT5 arginine methyltransferase in cancer cells. Science. 2016; 351(6278), 1214–1218.Kryukov GV, Wilson FH, Ruth JR et al. MTAP deletion confers enhanced dependency on the PRMT5 arginine methyltransferase in cancer cells. Science. 2016; 351(6278), 1214–1218. which may sensitize to inhibitors of PRMT5 [118]Kryukov GV, Wilson FH, Ruth JR et al. MTAP deletion confers enhanced dependency on the PRMT5 arginine methyltransferase in cancer cells. Science. 2016; 351(6278), 1214–1218.Kryukov GV, Wilson FH, Ruth JR et al. MTAP deletion confers enhanced dependency on the PRMT5 arginine methyltransferase in cancer cells. Science. 2016; 351(6278), 1214–1218..
Breaking down these components of resistance in a framework such as this facilitates their independent investigation via available multi-omics and clinical data either from clinical trials or real-world practice. However, the more significant power comes from then reintegrating them together and validating findings in the lab and clinic. This integration of knowledge across an incredibly complex biological system, can provide the clues for which combination therapies will be more effective across different indications and can help identify markers for the optimal selection of patients for treatment according to their underlying biology. In an era where big data is booming and expanding rapidly to encompass immunological as well as molecular measures, this outcome for patients may not be in a too far distant future.
Affiliations
Ross Stewart
Translational Medicine, Oncology R&D,
AstraZeneca, Cambridge,
UK
Martin L Miller
Oncology Data Science, Oncology R&D,
AstraZeneca, Cambridge,
UK
Ana Camelo
Oncology Data Science, Oncology R&D,
AstraZeneca, Cambridge,
UK
J Carl Barrett
Translational Medicine, Oncology R&D,
AstraZeneca, Boston, MA,
USA
References
1. McGranahan N, Furness AJ, Rosenthal R et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Sci. 2016; 351(6280), 1463–1469. Crossref
2. Anagnostou V, Smith KN, Forde PM et al. Evolution of Neoantigen Landscape during Immune Checkpoint Blockade in Non-Small Cell Lung Cancer. Cancer Discov. 2017; 7(3), 264–276. Crossref
3. Sade-Feldman M, Jiao YJ, Chen JH et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 2017; 8(1), 1136. Crossref
4. Rasmussen M, Durhuus JA, Nilbert M, Andersen O, Therkildsen C. Response to Immune Checkpoint Inhibitors Is Affected by Deregulations in the Antigen Presentation Machinery: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022; 12(1). Crossref
5. Chowell D, Krishna C, Pierini F et al. Evolutionary divergence of HLA class I genotype impacts efficacy of cancer immunotherapy. Nat. Med. 2019; 25(11), 1715–1720. Crossref
6. Montesion M, Murugesan K, Jin DX et al. Somatic HLA Class I Loss Is a Widespread Mechanism of Immune Evasion Which Refines the Use of Tumor Mutational Burden as a Biomarker of Checkpoint Inhibitor Response. Cancer Discov. 2021; 11(2), 282–292. Crossref
7. Yu S, Zhao Z, Chen L et al. HLA loss of heterozygosity-mediated discordant responses to immune checkpoint blockade in squamous cell lung cancer with renal metastasis. Immunother. 2021; 13(3), 195–200. Crossref
8. Yang Y, Kim E, Kim S. Insignificant effects of loss of heterozygosity in HLA in the efficacy of immune checkpoint blockade treatment. Genes Genomics. 2022; 44(4), 509–515. Crossref
9. Litchfield K, Reading JL, Puttick C et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell. 2021; 184(3), 596–614 e514. Crossref
10. Kwak Y, Koh J, Park Y et al. Differential prognostic impact of CD8(+) T cells based on human leucocyte antigen I and PD-L1 expression in microsatellite-unstable gastric cancer. Br J Cancer. 2020; 122(9), 1399–1408. Crossref
11. Hurkmans DP, Kuipers ME, Smit J et al. Tumor mutational load, CD8(+) T cells, expression of PD-L1 and HLA class I to guide immunotherapy decisions in NSCLC patients. Cancer Immunol. Immunother. 2020; 69(5), 771–777. Crossref
12. Jimenez-Sanchez A, Memon D, Pourpe S et al. Heterogeneous Tumor-Immune Microenvironments among Differentially Growing Metastases in an Ovarian Cancer Patient. Cell. 2017; 170(5), 927–938 e920. Crossref
13. Zhang AW, McPherson A, Milne K, et al. Interfaces of Malignant and Immunologic Clonal Dynamics in Ovarian Cancer. Cell. 2018; 173(7), 1755–1769 e1722. Crossref
14. Jimenez-Sanchez A, Cybulska P, Mager KL et al. Unraveling tumor-immune heterogeneity in advanced ovarian cancer uncovers immunogenic effect of chemotherapy. Nat. Genet. 2020; 52(6), 582–593. Crossref
15. Liu D, Lin JR, Robitschek EJ et al. Evolution of delayed resistance to immunotherapy in a melanoma responder. Nat. Med. 2021; 27(6), 985–992. Crossref
16. Spranger S, Gajewski TF. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat. Rev. Cancer. 2018; 18(3), 139–147. Crossref
17. Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 2020; 20(1), 25–39. Crossref
18. Chin YE, Kitagawa M, Su WC, You ZH, Iwamoto Y, Fu XY. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science. 1996; 272(5262), 719–722. Crossref
19. Ayers M, Lunceford J, Nebozhyn M, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest. 2017; 127(8), 2930–2940. Crossref
20. von Locquenghien M, Rozalen C, Celia-Terrassa T. Interferons in cancer immunoediting: sculpting metastasis and immunotherapy response. J. Clin. Invest. 2021; 131(1). Crossref
21. Benci JL, Xu B, Qiu Y et al. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell. 2016; 167(6), 1540–1554 e1512. Crossref
22. Lisberg A, Cummings A, Goldman JW et al. A Phase II Study of Pembrolizumab in EGFR-Mutant, PD-L1+, Tyrosine Kinase Inhibitor Naive Patients With Advanced NSCLC. J. Thorac. Oncol. 2018; 13(8), 1138–1145. Crossref
23. Papillon-Cavanagh S, Doshi P, Dobrin R, Szustakowski J, Walsh AM. STK11 and KEAP1 mutations as prognostic biomarkers in an observational real-world lung adenocarcinoma cohort. ESMO Open. 2020; 5(2). Crossref
24. Shire NJ, Klein AB, Golozar A et al. STK11 (LKB1) mutations in metastatic NSCLC: Prognostic value in the real world. PLoS One. 2020; 15(9), e0238358. Crossref
25. Zaretsky JM, Garcia-Diaz A, Shin DS et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016; 375(9), 819–829. Crossref
26. Shin DS, Zaretsky JM, Escuin-Ordinas H et al. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 2017; 7(2), 188–201. Crossref
27. Luke JJ, Bao R, Sweis RF, Spranger S, Gajewski TF. WNT/beta-catenin Pathway Activation Correlates with Immune Exclusion across Human Cancers. Clin. Cancer Res. 2019; 25(10), 3074–3083. Crossref
28. Lin Z, Huang L, Li SL, Gu J, Cui X, Zhou Y. PTEN loss correlates with T cell exclusion across human cancers. BMC Cancer. 2021; 21(1), 429. Crossref
29. Trujillo JA, Luke JJ, Zha Y et al. Secondary resistance to immunotherapy associated with beta-catenin pathway activation or PTEN loss in metastatic melanoma. J. Immunother. Cancer. 2019; 7(1), 295. Crossref
30. Ebot EM, Duncan DL, Tolba K et al. Deletions on 9p21 are associated with worse outcomes after anti-PD-1/PD-L1 monotherapy but not chemoimmunotherapy. NPJ Precis. Oncol. 2022; 6(1), 44. Crossref
31. Han G, Yang G, Hao D et al. 9p21 loss confers a cold tumor immune microenvironment and primary resistance to immune checkpoint therapy. Nat. Commun. 2021; 12(1), 5606. Crossref
32. Wang S, Jia M, He Z, Liu XS. APOBEC3B and APOBEC mutational signature as potential predictive markers for immunotherapy response in non-small cell lung cancer. Oncogene. 2018; 37(29), 3924–3936. Crossref
33. Yang H, Ma W, Sun B et al. Smoking signature is superior to programmed death-ligand 1 expression in predicting pathological response to neoadjuvant immunotherapy in lung cancer patients. Transl. Lung Cancer Res. 2021; 10(9), 3807–3822. Crossref
34. Pham TV, Boichard A, Goodman A et al. Role of ultraviolet mutational signature versus tumor mutation burden in predicting response to immunotherapy. Mol. Oncol. 2020; 14(8), 1680–1694. Crossref
35. Riaz N, Havel JJ, Kendall SM et al. Recurrent SERPINB3 and SERPINB4 mutations in patients who respond to anti-CTLA4 immunotherapy. Nat. Genet. 2016; 48(11), 1327–1329. Crossref
36. Okamura R, Kato S, Lee S, Jimenez RE, Sicklick JK, Kurzrock R. ARID1A alterations function as a biomarker for longer progression-free survival after anti-PD-1/PD-L1 immunotherapy. J. Immunother. Cancer. 2020; 8(1). Crossref
37. Liu C, Zheng S, Jin R et al. The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer Lett. 2020; 470, 95–105. Crossref
38. Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat. Immunol. 2022; 23(4), 487–500. Crossref
39. Galluzzi L, Lopez-Soto A, Kumar S, Kroemer G. Caspases Connect Cell-Death Signaling to Organismal Homeostasis. Immunity. 2016; 44(2), 221–231. Crossref
40. Zhang Z, Zhang Y, Xia S et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020; 579(7799), 415–420. Crossref
41. Zhou B, Abbott DW. Gasdermin E permits interleukin-1 beta release in distinct sublytic and pyroptotic phases. Cell Rep. 2021; 35(2), 108998. Crossref
42. Humeau J, Leduc M, Cerrato G, Loos F, Kepp O, Kroemer G. Phosphorylation of eukaryotic initiation factor-2alpha (eIF2alpha) in autophagy. Cell Death Dis. 2020; 11(6), 433. Crossref
43. Hou J, Zhao R, Xia W et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol. 2020; 22(10), 1264–1275. Crossref
44. Lv J, Liu Y, Mo S et al. Gasdermin E mediates resistance of pancreatic adenocarcinoma to enzymatic digestion through a YBX1-mucin pathway. Nat. Cell Biol. 2022; 24(3), 364–372. Crossref
45. Ning X, Wang Y, Jing M et al. Apoptotic Caspases Suppress Type I Interferon Production via the Cleavage of cGAS, MAVS, and IRF3. Mol Cell. 2019; 74(1), 19–31 e17. Crossref
46. Shi CS, Kehrl JH. Bcl-2 regulates pyroptosis and necroptosis by targeting BH3-like domains in GSDMD and MLKL. Cell Death Discov. 2019; 5, 151. Crossref
47. Erkes DA, Cai W, Sanchez IM et al. Mutant BRAF and MEK Inhibitors Regulate the Tumor Immune Microenvironment via Pyroptosis. Cancer Discov. 2020; 10(2), 254–269. Crossref
48. Eagle H. Nutrition needs of mammalian cells in tissue culture. Science. 1955; 122(3168), 501–514. Crossref
49. Stockwell BR. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022; 185(14), 2401–2421. Crossref
50. Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021; 18(5), 280–296. Crossref
51. Wang W, Green M, Choi JE et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019; 569(7755), 270–274. Crossref
52. Liao P, Wang W, Wang W et al. CD8(+) T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell. 2022; 40(4), 365–378 e366. Crossref
53. Kim R, Hashimoto A, Markosyan N et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature. 2022; 612(7939), 338–346. Crossref
54. Kepp O, Zitvogel L, Kroemer G. Clinical evidence that immunogenic cell death sensitizes to PD-1/PD-L1 blockade. Oncoimmunol. 2019; 8(10), e1637188. Crossref
55. Tumeh PC, Harview CL, Yearley JH et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014; 515(7528), 568–571. Crossref
56. Vanhersecke L, Brunet M, Guegan JP et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat. Cancer. 2021; 2(8), 794–802. Crossref
57. Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022; 12(1), 31–46. Crossref
58. Denton AE, Roberts EW, Fearon DT. Stromal Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2018; 1060, 99–114. Crossref
59. Kim BG, Malek E, Choi SH, Ignatz-Hoover JJ, Driscoll JJ. Novel therapies emerging in oncology to target the TGF-beta pathway. J. Hematol. Oncol. 2021; 14(1), 55. Crossref
60. Jung KY, Cho SW, Kim YA et al. Cancers with Higher Density of Tumor-Associated Macrophages Were Associated with Poor Survival Rates. J. Pathol. Transl. Med. 2015; 49(4), 318–324. Crossref
61. Christofides A, Strauss L, Yeo A, Cao C, Charest A, Boussiotis VA. The complex role of tumor-infiltrating macrophages. Nat. Immunol. 2022; 23(8), 1148–1156. Crossref
62. Duan Z, Luo Y. Targeting macrophages in cancer immunotherapy. Signal Transduct. Target Ther. 2021; 6(1), 127. Crossref
63. Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015; 27(4), 462–472. Crossref
64. Yu J, Green MD, Li S et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat. Med. 2021; 27(1), 152–164. Crossref
65. Li T, Liu T, Zhu W et al. Targeting MDSC for Immune-Checkpoint Blockade in Cancer Immunotherapy: Current Progress and New Prospects. Clin. Med. Insights Oncol. 2021; 15, 11795549211035540. Crossref
66. Gardner A, de Mingo Pulido A, Ruffell B. Dendritic Cells and Their Role in Immunotherapy. Front. Immunol. 2020; 11, 924. Crossref
67. Busa R, Bulati M, Badami E et al. Tissue-Resident Innate Immune Cell-Based Therapy: A Cornerstone of Immunotherapy Strategies for Cancer Treatment. Front. Cell Dev. Biol. 2022; 10, 907572. Crossref
68. Garris CS, Arlauckas SP, Kohler RH et al. Successful Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-gamma and IL-12. Immunity. 2022; 55(9), 1749. Crossref
69. Kantoff PW, Higano CS, Shore ND et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010; 363(5), 411–422. Crossref
70. Islam R, Pupovac A, Evtimov V et al. Enhancing a Natural Killer: Modification of NK Cells for Cancer Immunotherapy. Cells. 2021; 10(5). Crossref
71. Wong JKM, Dolcetti R, Rhee H, Simpson F, Souza-Fonseca-Guimaraes F. Weaponizing natural killer cells for solid cancer immunotherapy. Trends Cancer. 2022. Crossref
72. Chiossone L, Vivier E. Bringing natural killer cells to the clinic. J. Exp. Med. 2022; 219(10). Crossref
73. Bruno A, Focaccetti C, Pagani A et al. The proangiogenic phenotype of natural killer cells in patients with non-small cell lung cancer. Neoplasia. 2013; 15(2):, 133–142. Crossref
74. Lin M, Luo H, Liang S et al. Pembrolizumab plus allogeneic NK cells in advanced non-small cell lung cancer patients. J. Clin. Invest. 2020; 130(5), 2560–2569. Crossref
75. Herbst RS, Majem M, Barlesi F et al. COAST: An Open-Label, Phase II, Multidrug Platform Study of Durvalumab Alone or in Combination With Oleclumab or Monalizumab in Patients With Unresectable, Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022; 40(29), 3383–3393. Crossref
76. Ley K, Hoffman HM, Kubes P et al. Neutrophils: New insights and open questions. Sci. Immunol. 2018; 3(30). Crossref
77. Sionov RV, Fridlender ZG, Granot Z. The Multifaceted Roles Neutrophils Play in the Tumor Microenvironment. Cancer Microenviron. 2015; 8(3), 125–158. Crossref
78. Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 2019; 16(10), 601–620. Crossref
79. Salcher S, Sturm G, Horvath L et al. High-resolution single-cell atlas reveals diversity and plasticity of tissue-resident neutrophils in non-small cell lung cancer. Cancer Cell. 2022. Crossref
80. Arasanz H, Bocanegra AI, Morilla I et al. Circulating Low Density Neutrophils Are Associated with Resistance to First Line Anti-PD1/PDL1 Immunotherapy in Non-Small Cell Lung Cancer. Cancers (Basel). 2022; 14(16). Crossref
81. Pan C, Wu QV, Voutsinas J et al. Neutrophil to lymphocyte ratio and peripheral blood biomarkers correlate with survival outcomes but not response among head and neck and salivary cancer treated with pembrolizumab and vorinostat. Head Neck. 2022. Crossref
82. Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010; 29(8), 1093–1102. Crossref
83. Li F, Li C, Cai X et al. The association between CD8+ tumor-infiltrating lymphocytes and the clinical outcome of cancer immunotherapy: A systematic review and meta-analysis. EClinicalMedicine. 2021; 41, 101134. Crossref
84. Hammerl D, Martens JWM, Timmermans M et al. Spatial immunophenotypes predict response to anti-PD1 treatment and capture distinct paths of T cell evasion in triple negative breast cancer. Nat. Commun. 2021; 12(1), 5668. Crossref
85. Simoni Y, Becht E, Fehlings M et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018; 557(7706), 575–579. Crossref
86. Lowery FJ, Krishna S, Yossef R et al. Molecular signatures of antitumor neoantigen-reactive T cells from metastatic human cancers. Science. 2022; 375(6583), 877–884. Crossref
87. Jansen CS, Prokhnevska N, Master VA et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature. 2019; 576(7787), 465–470. Crossref
88. Gao J, Ward JF, Pettaway CA et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat. Med. 2017; 23(5), 551–555. Crossref
89. Tay RE, Richardson EK, Toh HC. Revisiting the role of CD4(+) T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene Ther. 2021; 28(1–2), 5–17. Crossref
90. Cohen M, Giladi A, Barboy O et al. The interaction of CD4(+) helper T cells with dendritic cells shapes the tumor microenvironment and immune checkpoint blockade response. Nat. Cancer. 2022; 3(3), 303–317. Crossref
91. Zhang Y, Chen H, Mo H et al. Single-cell analyses reveal key immune cell subsets associated with response to PD-L1 blockade in triple-negative breast cancer. Cancer Cell. 2021; 39(12), 1578–1593 e1578. Crossref
92. Kazanietz MG, Durando M, Cooke M. CXCL13 and Its Receptor CXCR5 in Cancer: Inflammation, Immune Response, and Beyond. Front Endocrinol (Lausanne). 2019; 10, 471. Crossref
93. Goswami S, Chen Y, Anandhan S et al. ARID1A mutation plus CXCL13 expression act as combinatorial biomarkers to predict responses to immune checkpoint therapy in mUCC. Sci. Transl. Med. 2020; 12(548). Crossref
94. Saleh R, Elkord E. FoxP3(+) T regulatory cells in cancer: Prognostic biomarkers and therapeutic targets. Cancer Lett. 2020; 490, 174–185. Crossref
95. Szabados B, Kockx M, Assaf ZJ et al. Final Results of Neoadjuvant Atezolizumab in Cisplatin-ineligible Patients with Muscle-invasive Urothelial Cancer of the Bladder. Eur. Urol. 2022; 82(2), 212–222. Crossref
96. Wouters MCA, Nelson BH. Prognostic Significance of Tumor-Infiltrating B Cells and Plasma Cells in Human Cancer. Clin. Cancer Res. 2018; 24(24), 6125–6135. Crossref
97. Cabrita R, Lauss M, Sanna A et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020; 577(7791), 561–565. Crossref
98. Helmink BA, Reddy SM, Gao J et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020; 577(7791), 549–555. Crossref
99. Griss J, Bauer W, Wagner C et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat. Commun. 2019; 10(1), 4186. Crossref
100. Patil NS, Nabet BY, Muller S et al. Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung cancer. Cancer Cell. 2022; 40(3), 289–300 e284. Crossref
101. Riaz N, Havel JJ, Makarov V et al. Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell. 2017; 171(4), 934–949 e916. Crossref
102. Valpione S, Mundra PA, Galvani E et al. The T cell receptor repertoire of tumor infiltrating T cells is predictive and prognostic for cancer survival. Nat. Commun. 2021; 12(1), 4098. Crossref
103. Kato T, Kiyotani K, Tomiyama E et al. Peripheral T cell receptor repertoire features predict durable responses to anti-PD-1 inhibitor monotherapy in advanced renal cell carcinoma. Oncoimmunol. 2021; 10(1), 1862948. Crossref
104. Cha E, Klinger M, Hou Y, et al. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci. Transl. Med. 2014; 6(238), 238ra270. Crossref
105. Kvistborg P, Philips D, Kelderman S et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci. Transl. Med. 2014; 6(254), 254ra128. Crossref
106. Kidman J, Principe N, Watson M et al. Characteristics of TCR Repertoire Associated With Successful Immune Checkpoint Therapy Responses. Front. Immunol. 2020; 11, 587014. Crossref
107. Han J, Duan J, Bai H et al. TCR Repertoire Diversity of Peripheral PD-1(+)CD8(+) T Cells Predicts Clinical Outcomes after Immunotherapy in Patients with Non-Small Cell Lung Cancer. Cancer Immunol. Res. 2020; 8(1), 146–154. Crossref
108. Jiang W, He Y, He W et al. Exhausted CD8+T Cells in the Tumor Immune Microenvironment: New Pathways to Therapy. Front. Immunol. 2020; 11, 622509. Crossref
109. Lopez de Rodas M, Nagineni V, Ravi A et al. Role of tumor infiltrating lymphocytes and spatial immune heterogeneity in sensitivity to PD-1 axis blockers in non-small cell lung cancer. J. Immunother. Cancer. 2022; 10(6). Crossref
110. Datar I, Sanmamed MF, Wang J et al. Expression Analysis and Significance of PD-1, LAG-3, and TIM-3 in Human Non-Small Cell Lung Cancer Using Spatially Resolved and Multiparametric Single-Cell Analysis. Clin. Cancer Res. 2019; 25(15), 4663–4673. Crossref
111. Awada G, Jansen Y, Schwarze JK et al. A Comprehensive Analysis of Baseline Clinical Characteristics and Biomarkers Associated with Outcome in Advanced Melanoma Patients Treated with Pembrolizumab. Cancers (Basel). 2021; 13(2). Crossref
112. Minichsdorfer C, Gleiss A, Aretin MB, Schmidinger M, Fuereder T. Serum parameters as prognostic biomarkers in a real world cancer patient population treated with anti PD-1/PD-L1 therapy. Ann Med. 2022; 54(1), 1339–1349. Crossref
113. Rounis K, Makrakis D, Tsigkas AP et al. Cancer cachexia syndrome and clinical outcome in patients with metastatic non-small cell lung cancer treated with PD-1/PD-L1 inhibitors: results from a prospective, observational study. Transl. Lung Cancer Res. 2021; 10(8), 3538–3549. Crossref
114. Cortellini A, Bersanelli M, Buti S et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J. Immunother. Cancer. 2019; 7(1), 57. Crossref
115. Xie M, Li N, Xu X et al. The Efficacy of PD-1/PD-L1 Inhibitors in Patients with Liver Metastasis of Non-Small Cell Lung Cancer: A Real-World Study. Cancers (Basel). 2022; 14(17). Crossref
116. Wang X, Ji Q, Yan X et al. The Impact of Liver Metastasis on Anti-PD-1 Monoclonal Antibody Monotherapy in Advanced Melanoma: Analysis of Five Clinical Studies. Front. Oncol. 2020; 10, 546604. Crossref
117. Nosrati A, Tsai KK, Goldinger SM et al. Evaluation of clinicopathological factors in PD-1 response: derivation and validation of a prediction scale for response to PD-1 monotherapy. Br. J. Cancer. 2017; 116(9), 1141–1147. Crossref
118. Kryukov GV, Wilson FH, Ruth JR et al. MTAP deletion confers enhanced dependency on the PRMT5 arginine methyltransferase in cancer cells. Science. 2016; 351(6278), 1214–1218. Crossref
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: The authors thank Deborah Shuman for her assistance with figure preparation.
Disclosure and potential conflicts of interest: Stewart R, Miller ML, Camelo A & Barrett JC report employment and stock ownership with AstraZeneca. The authors declare no other conflict of interests.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Immuno-Oncology Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2023 AstraZeneca. Published by Immuno-Oncology Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Mar 17 2023; Revised manuscript received: May 16 2023;
Publication date: May 26 2023
