Key considerations of cell and gene therapy cold chain logistics
Cell Gene Therapy Insights 2016; 2(2), 223-236.
10.18609/cgti.2016.025
While cell and gene therapy products share the same goal for their cold chains as standard pharmaceutical and biological products, namely to ensure the product is maintained within appropriate temperature specifications en route to the patient, there are specific features of cell therapy products that make their cold chains uniquely challenging. This article reviews the requirements of cold chains for these products, highlights some of the current challenges and discusses some of the technologies being developed to facilitate the creation of commercial cold chains for these advanced medicinal products.
Background
The cold chain for a medicinal product – be it a standard pharmaceutical or advanced medicinal product like a cell or gene therapy – is the sequence of transportation events required to maintain temperature within approved specifications from manufacture of the formulated bulk material through to the packaged product at the end user. Cold chains for the distribution (shipping/storage) of pharmaceuticals and biologic products have been well-established for decades, using platform processes and technologies to ensure efficient product storage and distribution depending on the products temperature requirements, whether it be room temperature, refrigerated or frozen (usually 0 to -40°C).
Cell therapy products are unique in that they consist of viable mammalian cells: typically human cells that are autologous or allogeneic. Hence, the cold chain for a cell therapy product must be capable of maintaining a living product in a viable state throughout storage and distribution, all the way to administration to the patient – a uniquely challenging criterion. Compared to small molecule pharmaceuticals and biologics, cells are highly labile, remaining viable only within narrow ranges of time and temperature. Cells also require oxygen and nutrients when metabolically active. Therefore, cell therapy products require either just-in-time delivery to patients, or cryogenic storage temperatures to preserve the cells in a metabolically inactive state. Currently there is a lack of existing cold chain infrastructure to meet these requirements, particularly from a commercial supply chain perspective.
On the other hand, gene therapy products consist of nucleic acids packaged in variety of different forms: viral vectors, inorganic complexes, or naked DNA/RNA. As these products are inherently more stable than cells, their storage and distribution can typically leverage approaches taken for conventional pharmaceuticals and biologicals. Hence, cold chains for gene therapy products do not typically present the same challenge as for cell therapies, and this discussion will focus primarily on challenges associated with cold chain distribution of cell therapy products.
Table 1 lists some examples of cell therapy products and their storage and/or shipping temperature requirements. Cell therapy products are typically distributed under controlled room temperature or refrigerated conditions.
| Table 1. Storage and/or shipping conditions of some cell therapy products. | ||
|---|---|---|
| Product (manufacturer) | Product Type | Storage and/or Shipping Temperature |
| Apligraf® (Organogenesis) | Allogeneic Cell Therapy | 68°F to 73°F (20-23°C) |
| Gintuit™ (Organogenesis) | Allogeneic Cell Therapy | 68°F to 73°F (20-23°C) |
| Carticel® (Vericel) | Autologous Cell Therapy | Room temperature |
| PROVENGE® (Dendreon) | Autologous Cell Therapy | Store the bag in the insulated container to maintain the correct storage temperature (28°C) until infusion. Do not refrigerate or freeze the container |
| ChondroCelect® (TiGenix) | Autologous Cell Therapy | 15-25°C |
| Holoclar® (Holostem) | Autologous Cell Therapy | 15-25°C |
At present there are a limited number of commercially available cell therapy based products, however there are currently hundreds of cell therapies in registered clinical trials [1] with the anticipation that there will be numerous commercial products in the future. The commercial cold strategy for these products will depend on product stability and product distribution approach. Typical temperature ranges relevant for cell therapy products are either i) controlled room temperature (15–25°C), or refrigerated (2–8°C), both of which require just-in-time delivery of the cells to patients post manufacture of the cell product, or ii) cryogenic temperature (vapor phase of liquid nitrogen [LN2]) for cryogenically preserved cells. The advantages and disadvantages of both approaches are discussed below.
General Cold Chain Requirements
Understanding product stability under storage/distribution conditions
Whether a cell therapy product is shipped at room temperature, refrigerated, or under cryogenic conditions, data are required to understand the product stability as a function of temperature and storage time. Data should be generated both during development and from quality control (QC) stability studies using appropriate assays to monitor indications of product stability. These data are used to set time and temperature ranges appropriate for the product during transit and storage.
It is important to generate data to support the stability of the product over the range of temperatures that the product will experience during distribution. For example, a cryogenically frozen product may be shipped for up to 2 weeks in the vapor phase of LN2 to a clinical site, stored in a -80°C freezer for up to 2 days, transported to a surgery suite on dry ice, thawed and held at room temperature for an hour, and then administered to a patient. Even with very well designed storage and shipping systems, transient warming events as products are transferred between storage containers are often inevitable. Refrigerated vials will warm slightly when shipping boxes are opened in customs whilst cryogenically stored vials will warm as they are transferred from a cryoshipper to a long term cryo-storage container. Data to confirm that product quality is not impacted by one or many short term warming events, each lasting maybe a couple of minutes, are needed in order to design storage and shipping equipment and define shipping and transfer procedures. Due to the labile nature of cell therapy products in particular and the time sensitivity in handing cells, it is important to establish clear and practical guidance for clinical sites to ensure correct product handling.
Qualification of shippers
At a minimum, shippers should be qualified to maintain the product within the specified temperature ranges up to a qualified shipping time.
Development testing
Development testing should be performed before performance qualification testing to determine the appropriate insulated shipping container and the packing configuration of refrigerant (pack-out) and product. Packing configurations for minimum and maximum loads and the type and amount of refrigerant must be specified. These tests should be conducted using ambient testing profiles that are representative of the temperature conditions that the shipper may encounter in transit. Tests may also be used to determine shipper capabilities when exposed to adverse conditions for which the shippers were not designed. The duration of the thermal tests should exceed what is expected for routine shipments. The goal should be to develop a configuration for each shipping container that can be used year round.
Simulated Distribution Test
This test should be performed to confirm the ability of the shipping container to protect its contents from damage. The assembled package is tested according to transit industry standard test methods (e.g., ASTM D4169). This testing comprises a series of drop, vibration, and compression tests. Following testing, shipping packages are visually examined to confirm they are free from damage and the insulated container still provides acceptable thermal and physical protection to the contents.
Route verification
Route verification consists of performing a mock shipment between the shipping and receiving sites to confirm acceptable performance and temperature monitoring data during shipment can be obtained. This can also be used to evaluate the shipping logistics and receiving procedures. Route verification should take into account variations in transit temperatures depending on location or time of year (e.g., Arizona in the summer vs. Moscow in the winter).
Monitoring of product during shipment
Even after a shipping container and route have been qualified, to confirm the shipment does not exceed the qualified duration nor is exposed to some unforeseen extreme condition, it may be beneficial to monitor all shipments using portable temperature recorders . The appropriate position for the temperature monitor within the shipper should be determined during development and qualification of the shipper.
Ensuring product is controlled to prevent mix-ups & maintain traceability
Processes need to be developed to prevent mix-ups, contamination, and cross-contamination of products, supplies and reagents and to prevent products from improperly being made available for distribution. The process includes the selection, purchase, receipt, label inventory verification, and the steps taken to ensure that all products are labeled in compliance with requirements. Containers must follow 21 CFR 1271.200 regulations to prevent cross contamination.
Container closure integrity
Packaging and shipping containers must be designed and constructed to protect the product from contamination, and testing should be performed to demonstrate the suitability of the primary container to meet its intended use (maintenance of product quality during its shelf life). Container Closure Integrity (CCI) studies for the primary containers should include verification that the container closure remains intact after exposure of the primary container to transport conditions. Dye penetration in the presence of a pressure differential is an example of one test method for CCI challenge. Testing of transport conditions may be performed by either an actual shipment via the proposed shipping route or through a controlled study that simulates shipment conditions. The shipping simulation study must include “real-world” representative stress factors such as vibration, dropping, and exposure to pressure changes.
Receipt/inspection
Sites receiving products should have procedures which require the evaluation of shipping containers and the shipped materials for observable physical damage, verification of temperature monitor seal checks, time restrictions (such as time out of refrigeration during unpacking), temperature data monitor termination, reading, and interpretation, and must require verification of acceptable shipping conditions.
Additional Cold Chain Distribution Considerations for Cell Therapies
Just-in-time delivery
Many cell therapy products are stored and shipped unfrozen (at controlled room temperature or refrigerated temperatures). This is particularly true of current marketed cell therapy products. Because cells are metabolically active (albeit at a reduced level under refrigeration) their expiration period is typically on the order of days due to oxygen and nutrient limitations. Expiration date can be extended to several weeks by increasing the volume of storage medium, by reducing the storage temperature, or by attaching a series of bags or compartments that allow the medium to be exchanged without breaching the sterility of the system. These products are shipped in insulating containers designed and configured with appropriate refrigerant (gel packs/bricks/bottles, dry ice, or LN2) to provide the temperature environment required by the material being shipped and for a period of storage at the clinical site. The insulated containers must also protect the integrity of the packaged product.
A major disadvantage to this approach lies in the time-sensitive nature of the product at higher-than-cryogenic temperature. Because the cell product cannot be stored for any appreciable time period, the schedule for product manufacturing is bound to the timing for product administration to the patient. Any delays in product manufacturing means patients do not get their treatments on time, and conversely any changes in availability of patients for treatment may mean manufactured product is discarded.
Cryogenic preservation
Cryopreservation is the main mode used for the long-term storage of cells and has many advantages. It can vastly extend product shelf life compared to ambient or refrigerated storage (i.e., years of storage compared to days of storage). Importantly, it decouples the manufacturing schedule from the procedure wherein the cells are administered to the patient. As cells can be frozen almost indefinitely at cryogenic temperatures below the glass transition point of water, the manufacturing of the cells does not have to be coordinated to deliver product just in time for the clinical use of the cells. It also allows decoupling of testing of the product from the clinical use of the cells, enabling assays that require extended time (for example a 14 day sterility test) to be completed prior to batch release to the market. Finally, as cells are no longer stored in a metabolically active state, cell growth media can be removed from final product formulation and eliminating dosing unnecessary components to patients. Cryogenic preservation is typically enabled by storage of cells in the vapor phase of LN2. Dry ice or ultra-low temperature freezers may provide storage at warmer temperatures, circa -80°C, in cases where cells are stable for short time periods at these temperatures.
Traceability
There must be a system in place for a Human Cells, Tissues and Cellular and Tissue-based Product (HCT/P) that enables their tracking from the donor to the consignee or final disposition; and from the consignee or final disposition to the donor. The system shall be developed to control for contamination or cross contamination and facilitate the investigation of an actual or suspected transmission of a communicable disease and to take appropriate and timely corrective action. Each HCT/P manufactured must be assigned and labeled with a distinct identification code, that relates the HCT/P to the donor and to all records pertaining to the HCT/P, 1271.290 (e) FDA regulations.
Formulation
Cells shipped for just in time delivery are formulated with media components to provide nutrients to the cells. Cells cryogenically frozen are typically formulated with novel excipients to help the cells survive the freezing and thawing processes. Typically not all of these novel excipients are available in compendial grade. The formulation may also contain animal-derived components such as human serum albumin or fetal serum albumin which are less favorable from a viral or prion risk perspective, and require extensive diligence and supplier quality efforts to ensure safety. Dimethyl sulfoxide (DMSO) is an example of an excipient that is frequently used in cryopreservation formulations to maintain cell viability during final product freezing and thawing. DMSO is available that conforms with USP, PhEur and is used in a number of medical applications [2]. There is debate as to whether DMSO should be used in formulations as it is thought to be toxic. In general it is advisable to reduce the amount of DMSO as much as possible as this may improve the viability of product stability before and after thaw and also may reduce the potential toxicity that could occur during administration. However, DMSO can be used safely. Some of the loss of viability of cells in cryomedium can also be attributed to lack of metabolites, controlled temperature and dissolved gasses. It is the responsibility of the cell therapy product manufacturer to demonstrate safety and the utility of the excipients for their application, and route of administration. An additional consideration with the use of organic solvents such DMSO is the potential for higher levels of leachates from single use formulation equipment, or from the primary package. Formulation boundary studies can be used to determine minimal levels of excipients needed to maintain formulation utility and hence limit patient exposure.
Radiation risk
When considering the risks of shipping cell therapy products by aircraft the risk of radiation is often raised because of the potential damage to DNA of living cells. There are two types of radiation to consider: cosmic radiation while in flight and x-ray during security screening.
Cosmic radiation is a mixture of high energy particles originating from outside our solar system and also from our sun. Our atmosphere shields us from the potentially dangerous higher energy particles. Cosmic radiation is increased with altitude. Flying 12 Km (39,000 ft) high, passengers and crews of jet airliners are exposed to at least 10 times the cosmic ray dose that people at sea level receive (Evaluation of the Cosmic Radiation Exposure of Aircraft Crew A background to aircrew dose evaluation with results reported within the EC contract FIGM-CT-2000-00068 [DOSMAX], work package 6). However, the minimal increase in cosmic radiation exposure during air freight shipping is not a risk that is significant for cell therapy products.
Repeated x-ray exposure is associated with increased chromosomal instability and cancer. There is therefore a theoretical risk to cell therapy products. Organ transplants and hematopoietic stem cells transportation containers are usually labeled with a statement such as Do NOT X-Ray. However, there is limited data to support the notion of deleterious effects of transportation related x-ray exposure on human tissues. One of the few studies performed concludes that repeated exposure to the low radiation dose of hand-luggage control systems (1.5 ± 0.6 μSv per exposure) seems to be harmless for hematopoietic stem cells [3]. The radiation dose typically received by objects screened by the x-ray cabinets used for airport freight security is 1 millirad or less. The average dose rate from background radiation is 360 millirad per year [4].
Container closure at cryogenic storage temperatures
While containers must be designed and constructed to protect the product from contamination, recent studies [5] have demonstrated that container closure integrity of vialed products can be lost while at cryogenic temperatures (e.g., in ultra-low temperature freezers, on dry ice, or in LN2 freezer). This is due to the shrinkage of the vial stoppers and loss of elasticity at cryogenic temperatures. Although vialed product containers may be integral at room temperature before and after cryogenic storage, gas exchange into the headspace of the vial can occur during cryogenic storage. This can lead to build-up of CO2 or N2 in the vial, which can lead to over-pressure in the vial when the vial regains integrity after it is returned to ambient conditions. Due to these container closure integrity issues, if LN2 dewars are used, vials should be stored in the vapor phase and not the liquid phase to minimize any possibility of cross contamination between products stored within the same dewar. For products stored or shipped cryogenically on dry ice, the ingress of CO2, due to loss of container integrity at cryogenic temperatures, or gas diffusion through thin polymer layers, can result in CO2 absorption into the product and subsequent decrease in formulation pH after thaw, as shown in Figure 1.
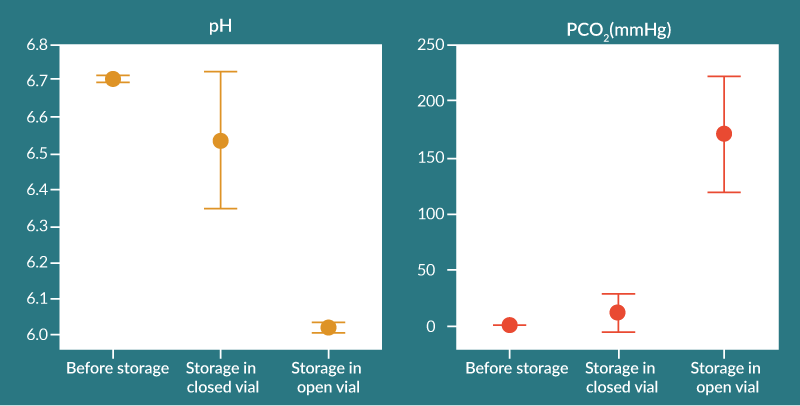
Figure 1. Effect of 2 weeks dry ice storage on pH and pCO2 of cell therapy product stored in a stoppered vial.
Labeling
To maintain traceability, cell and gene therapy products must be labeled. For products that require just in time delivery, labeling is performed soon after filling. For cryogenically preserved products, labeling typically occurs after the product is filled but before freezing, as labeling after the product is frozen requires either (1) labeling under cryogenic conditions or (2) exposing the frozen product to a significant transient warming event during the labeling operation. Labeling immediately after filling is also challenging, as labeling must typically be performed quickly, limiting the cure time of the labels at ambient conditions prior to cryogenic freezing. Poor label application or reduced label cure time can lead to label defects (peeling labels, labels falling off) after storage of the labeled product in cryogenic temperatures.
For cryogenically preserved vials, alternatives to consider to the traditional direct application of paper/plastic labels to the primary package include fitting of dog-tags to vials or permanently affixing vial holders to the vials, both of which can contain the same information as a traditional label. Both of these methods can in theory be performed under cryogenic conditions, which allows decoupling of vial filling and labeling operations, allowing cryogenically frozen product to be stored for long periods and where new data becomes available to support longer expiry dating, the product can be labeled with the latest product expiry date immediately prior to shipping. Additionally, portions of a batch can be separately labeled with regional specific information depending on where the product is being shipped.
Elements of Cryogenic Cold Chain Technology
Controlled room temperature and refrigerated cold chain technologies are well established and can be leveraged from existing systems in place for biopharmaceuticals. We will therefore focus our discussion on cold chain technologies for cell therapies on cryogenic technologies as these cryogenic cold chains are not yet widely established. The ability to store cell therapy products long term, which can only be provided by cryogenic storage, is an obvious goal for the future state of the industry, allowing the decoupling of manufacturing and clinical activities and eliminating the just-in-time nature of the current commercial distribution model. Unfortunately, the technological and commercial barriers to establishing an economical cryogenic cold chain distribution model for cell therapies presents a significant challenge.
LN2 storage
The most common type of storage freezer for cryopreservation utilizes LN2 filled into the bottom of a storage chamber, which is typically a cylindrical containment vessel of stainless steel construction. The resulting vapor phase nitrogen fills the rest of the storage chamber, where the product is held in racks above liquid level. Freezers of this design are typically qualified to maintain a storage temperature range of -190 to -120°C, with the warmest temperatures at the top of the vessel. Where very tight control of the product or sample temperature is required, typically the usable storage space within the freezer is constrained from top to bottom to minimize the variation between samples. There is typically no physical separation of the liquid level from the vapor phase, so the product must be handled properly to ensure it is not submerged or soaked by the LN2. Direct submersion in or contact with LN2 is typically avoided to prevent ingress of the liquid as a route of sample contamination. While these freezers are commonly mounted on casters, movement of freezers containing product or samples must be done carefully to avoid sloshing of the LN2 at the bottom of the chamber and making contact with the product. Whereas the typical LN2 storage freezer can exhibit large temperature gradations with its warmest location at the top of the chamber and its coldest locations at the bottom, just above the LN2, an isothermal design seeks to achieve a uniform temperature throughout the chamber. In this design, the LN2 is held in an annular cavity (jacket), which then cools the atmosphere within the storage chamber. In this manner, there is much less variation in temperature within the storage chamber from top to bottom. Also this design is inherently “dry”, protecting the product from ever experiencing direct contact with the LN2. This means less risk of liquid ingress or cross-contamination and also makes the freezer design better for transport. LN2 storage freezers are the only freezers capable of achieving storage temperatures as low as -190°C. This ultra-low storage temperature is desirable because it mitigates product temperature excursions when the freezer is accessed or when product is moved through ambient temperatures. The lower the product temperature, the more time it will take for the product to reach a critical warming temperature (i.e., above its glass transition temperature). Another advantage of the LN2 freezer class is its ability to hold temperature in the event of a power failure or failure of the LN2 distribution system. Depending on the level of LN2 in the freezer, these types of units can hold cryogenic temperatures for days without power or resupply of LN2.
Ultra-cold mechanical freezers
For applications where supply of LN2 is not readily available or convenient, there are mechanical freezers capable of maintaining temperatures as low as -150°C. The design of these freezers is typically “chest style” with the product stored in the bottom portion of the chamber. This design supports better uniformity of the product storage temperature and helps to mitigate excursions when the freezer door is opened. These freezers require a constant power supply in order to maintain temperatures and the warming rate in the event of a power failure is much faster than for a LN2 storage freezer.
Dry shippers
Dry shippers are designed with anterior walls to hold an absorptive cooling medium and an interior chamber to hold the product. To “charge” the shipper prior to use, the medium is soaked in LN2 to its maximum absorptive capacity before excess LN2 is removed. In its ready state, vapor phase nitrogen sublimates off the adsorptive medium containing the LN2, providing the cooling capacity of the shipper. The product is held in an internal cavity which is separated by a porous wall – this allows the vapor off-gassing from the medium to continuously cool the air in the cavity surrounding the product. Dry shippers come in a variety of sizes, with numerous options for shipment of 1000 vials or less (depending on the container size). In recent years, customized dry shippers have been developed by palletizing large storage freezers and modifying them for use with the dry absorptive medium. These units can accommodate on the order of 20,000 vials. However, there are not many options in between these small and large scale solutions. In that regulations restrict the total amount of hazardous liquids (e.g., LN2) on aircraft, dry shippers were designed for use in air transit and thus provide a compliant, safe solution for cryo shipping via aircraft. Pros: Dry shippers exhibit no risk of LN2 coming into direct contact with product; mitigating concerns with damaging product or cross-contamination. Because the shipper complies with flight regulations, it can be handled by major carriers. Dry shippers come in a variety of sizes, with numerous options for shipments of 1000 vials or less (depending on the container size). Cons: The orientation of the dry-shipper is a critical factor impacting the hold time at cryogenic temperatures. When the shipper is not in an upright position, the rate of vapor loss rapidly increases, thereby shortening the remaining hold time for the product. There are limited options for bulk shipment of large quantities of vials in dry shippers (>1000 vials).
Palletized storage freezers
For shipping and transport applications where the standard dry shipper options do not provide the desired capacity, there are specialist vendors who provide modifications of cryogenic storage freezers to make them shippable. For these applications, it is important that the design sufficiently contains the LN2 protecting the product from direct contact and preventing spillage of LN2 during transport.
Mobile storage freezers
For the movement of large quantities or entire inventories of frozen product, there are freezers designed with transport in mind. Most freezers are equipped with casters for portability. The movement of standard storage dewars, however, where LN2 is held in the same chamber just below the product, introduces a risk of LN2 sloshing and making direct contact with the product. The temperature distribution within the freezer could also potentially be affected as a result of this transport activity. Alternatively, the isothermal design of storage freezers separates the internal storage chamber for product from the annular space containing the LN2 cooling medium. With this design, there is less risk of product or samples coming into direct contact with the LN2 when in motion.
Sample carriers
For the local transport of frozen product or samples within a facility or hospital campus, small portable carriers with passive temperature control are available to accommodate standard-size cryo boxes. These insulated carriers leverage LN2 for cooling capacity, with a separate chamber to hold product, keeping them from direct contact with the LN2 during use. Some carrier units are equipped with built-in temperature monitoring and audible alarm capabilities notifying users in real-time if the chamber is warming above the desired limits. These carriers are compact and light-weight, even when fully charged with LN2, such that they can be transported manually and stored bench-top.
Portable Cryo-Workbench
After the initial cryopreservation step, subsequent handling or manipulation of the frozen product may be required. Depending on the duration of these manipulations and the critical temperature limits of the product, it may be necessary to perform manipulations in an ultra-cold or cryo environment. For these applications, portable cryo-workbenches are available which provide an intermediate transport vessel and work space with passive temperature control to limit the extent of product warming when removed from storage. These cryo-workbenches are designed with a raised platform for handling product within the insulated chamber and beneath which is a refillable reservoir of LN2 keeping the chamber cooled. The lids of the workbench can be removed to perform manipulations within the chamber, or closed to provide better insulation in between operations or during transport. An example of one application of the cryo-workbench is to aid in the preparation of frozen material for shipments, allowing users to pull quantities of frozen product from bulk storage racks and package for shipment. Additionally, the cryo-workbench can be leveraged for further downstream processing of frozen product, such as secondary packaging, or aid in the handling of products during sampling or investigations.
Commercialization Challenges
While the principles of cold chain logistics already established for established medicinal products (small molecule pharmaceuticals, biologics and vaccines) apply to the storage and distribution of cell and gene therapy products, due to the unique stability profile of cell-based products, the practical implementation of a cold chain distribution for cell-based products is a major challenge and is yet to be fully established on an industry-wide scale, leaving a significant burden on the developer of the cell therapy-based products to distribute their product.
Commercial distribution models & scale
Cell therapies are at the forefront of many new medicinal discoveries and will play an integral role in the roll-out of personalized medicine. While clinical trials are controlled and involve a relatively small distribution footprint, commercialization of cell therapies that will bring a new set of challenges requiring a “from-scratch” mind-set. The solutions and partners involved in the process have the potential to disrupt the traditional approach of biopharmaceutical distribution & channel management. Programs which will break the commercial barrier first will likely require custom/non-standard solutions as this category of medicines comes forth to a more mainstream status. As these first-in-industry programs approach commercial readiness, the question around distribution and channel management needs to be considered early as it may be necessary to introduce some elements within clinical studies. Some topics that require careful consideration include the following:
Labeling & packaging design
Labeling and packaging strategy must be able to with-stand storage conditions. For situations that require cryo-temperature storage, the label and package must be designed to support the unique manufacturing and storage process conditions. If product is stored in cryo conditions, package and label content may not be readable during storage and should be designed to take this into account. Additionally, the label will need to support the specified product thaw process. This is especially the case when a liquid based thaw is expected.
Shipping container selection
The shipping containers must be capable to hold product in acceptable storage conditions until the time of usage. In many circumstances, the shipping container will be used to store the product up through administration. For high volume products, it may require more permanent storage systems at the clinical site. Throughout the storage process, data logging of critical process parameters such as temperature and container orientation monitoring will need to be considered. Development of racking systems to support safe transport will also need to be designed.
Logistics providers
Product handoffs and transfers should be minimized based on the temperature sensitivity of the product. Partners who touch the product need to be familiar with, and capable of, maintaining appropriate storage conditions. While some logistics partners/segments have experience with the cryo-storage category, the majority do not and will require capital and time before being considered mainstream commercial options. All-inclusive-type logistics models allow enhanced levels of traceability along with the opportunity to minimize exception events from impacting product quality. Of course, this level of service comes with a significant Cost-Of-Goods impact. As cell therapy becomes more mainstream, shipments that require more custom delivery solutions will become harder to justify. The challenge for the industry is to build the requirements of cryogenic storage within their standard options.
Product transportation
Transporting product between third-party logistics providers, manufacturing partners, transportation carriers, and of course the customer require thought into the appropriate level of traceability and transfer of ownership. While some situations can use existing infrastructure and process, others will require custom handling solutions.
Inventory strategy
Because of challenging storage conditions and product complexities just-in-time type inventory models will likely be leveraged. As many of these products require patient scheduling, back-up supplies need to be considered.
Tracking & tracing
Industry is evolving to a more proactive model where shipments themselves will have the ability to regularly report on temperature, location, and package orientation as well as other critical variables related to shipments and allow corrective actions to resolve any concerns before they impact product quality. GPS tracking capabilities will enhance the shipper and receiver’s ability to track container location and provide tracking and delivery info on demand.
Clinical site storage
Current on-site storage capabilities vary by class of trade with larger hospitals and institutions offering more advanced temperature controlled storage. Region also plays a role in available capabilities. Most hospital pharmacies do not have capabilities to store products requiring ultra-cold chain conditions and will require custom solutions. LN2 freezers require planning and are not likely to be options for smaller customers or customers with limited facility and infrastructure. Usage of cryo shipping dewars as storage units is sufficient for just in time shipments that are consumed shortly after being received (within approximately 7 days).
Combination product complexity
Cell therapies may require customized delivery devices specifically designed for a particular product. In this situation, delivery devices may need to be stored in conditions that differ from the cell therapy product, e.g., at ambient temperature.
Conclusion
The just-in-time distribution model for current cell therapy products that utilizes controlled room temperature or refrigerated shipment of cells and couples cell therapy manufacturing to clinical administration of the product to the patient has major drawbacks as a commercial distribution model. Alternatively, a cold chain capable of maintaining cryopreservation conditions throughout distribution is not yet established. The practical implementation of a cold chain distribution for cell-based products is a major challenge and is yet to be fully established on an industry-wide scale, leaving a significant burden on the developer of the cell or gene therapy based-products to distribute their product.
Financial & competing interests disclosure
The authors have no relevant financial involvement with an organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock options or ownership, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License.
References
1. Trounson A, McDonald C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2002; 17, 17(1), 11–22. CrossRef
2. Swanson BN. Medical use of dimethyl sulfoxide (DMSO). Rev. Clin. Basic Pharm. 1985; 5(1–2), 1–33. Website
3. Petzer AL, Speth H-G, Hoflehner E et al. Breaking the rules? X–ray examination of haematopoietic stem cell grafts at international airports. Blood 2002; 99: 4632–3. CrossRef
4. Cabinet X-ray systems Website
5. Zuleger B, Werner U, Kort A et al. Container/Closure Integrity Testing and the Identification of a Suitable Vial/Stopper Combination for Low-Temperature Storage at -80°C. PDA J. Pharm. Sci. Technol. 2012; 66, 453–65. CrossRef
Affiliations
Francis Meacle1, Jeff Salkin2,Meredith Rice2 & Ian Harris1
11Cell Therapy, Janssen R&D, 1400 McKean Road, Spring House, PA 19477, USA
2Janssen Supply Chain