Immuno-Oncology Insights has ceased publication. However, all published content remains available here. |
Immuno-Oncology Insights was an open access, independently peer reviewed publication specifically designed to fill a number of clear and important gaps in the slate of journals for the industrial and academic and immuno-oncology communities.
Guided by an editorial board lead by Jon Wigginton and Renier J Brentjens, Immuno-Oncology Insights placed R&D and manufacturing challenges and progress across a wide variety of technology fields in context.
To find out more or to access some of our fantastic content, visit Immuno-Oncology Insights.
November 2024
Latest Articles

Why choose a modular, automated approach to autologous CAR T manufacturing?
28 May

Register for Bioconjugation Insights
7 May
INTERACTIVE EVENT SUMMARY: Harnessing flow cytometry for high-throughput screening in immunotherapy development
20 January

Spatial mapping: shaping the future of I-O diagnostics and treatment
Banafshé Larijani
6 December 2024
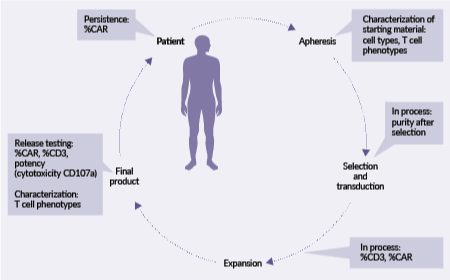
Navigating the opportunities and challenges in analytical development of flowcytometry for T cell therapy
Therese Choquette, Minh Ngoc Duong
25 November 2024

Revolutionizing the treatment of cancer with allogeneic CAR-T cell therapy
Cokey Nguyen
13 November 2024

Advancing cellular therapies: moving beyond the CAR-T landscape
Frank Borriello
5 September 2024
Cellular immunotherapy—a matter of delivery?
Nina Bauer
5 September 2024

Challenges and innovations in patient selection, biomarkers, and personalized treatments within the I-O landscape
Oliver Rosen
4 September 2024



