Bridging the translational gap: humanized liver models as predictive tools for RNA therapeutic success
Nucleic Acid Insights 2024; 1(9), 147–158
DOI: 10.18609/nai.2024.020
RNA therapeutics, particularly siRNAs, are advancing drug development, but traditional animal models often fail to predict human responses, leading to clinical trial failures. This article explores humanized liver chimeric mouse models, such as the PXB-mouse, as a solution to improve preclinical predictions of siRNA efficacy and safety. It highlights the limitations of conventional models and demonstrates, through case studies in viral hepatitis, metabolic disorders, and lipid regulation, how PXB-mice address challenges like off-target effects and therapeutic efficacy. These models offer a more human-relevant platform, potentially reducing late-stage failures and accelerating RNA therapeutic development. The PXB-mouse model features a liver that is highly engrafted with human hepatocytes, while other cell types remain of mouse origin—a factor to consider when assessing RNA therapeutics. Despite this limitation, humanized liver mice can provide greater confidence in the translational potential of investigational therapeutics than conventional animal models alone.
The field of RNA therapeutics, with its potential for treating a wide range of diseases, continues to experience rapid growth and attracts significant investment. According to the American Society of Gene and Cell Therapy (ASGCT), as of Q2 2024, 30 RNA therapies have been approved globally and another 1,125 are currently in development (between preclinical and pre-registration stages) [1]American Society of Gene and Cell Therapy and Citeline. Gene, Cell, and RNA Therapy Landscape Report. 2024. .
RNA therapeutics encapsulate several therapeutic modalities, including small interfering RNA (siRNA), messenger RNA (mRNA), and antisense oligonucleotides (ASO) (Table 1). IQVIA Pipeline Intelligence [2]Lutzmayer S, Wright A. IQVIA pipeline intelligence. IQVIA Nov 27, 2023. noted that these three types dominate the development landscape, collectively representing 80% of the RNA therapeutics pipeline. Among these, siRNA is the most prevalent category from preclinical to pre-registration stages accounting for 37% of the pipeline. The range of therapeutic indications is very broad, with rare diseases topping the list of targets (Figure 1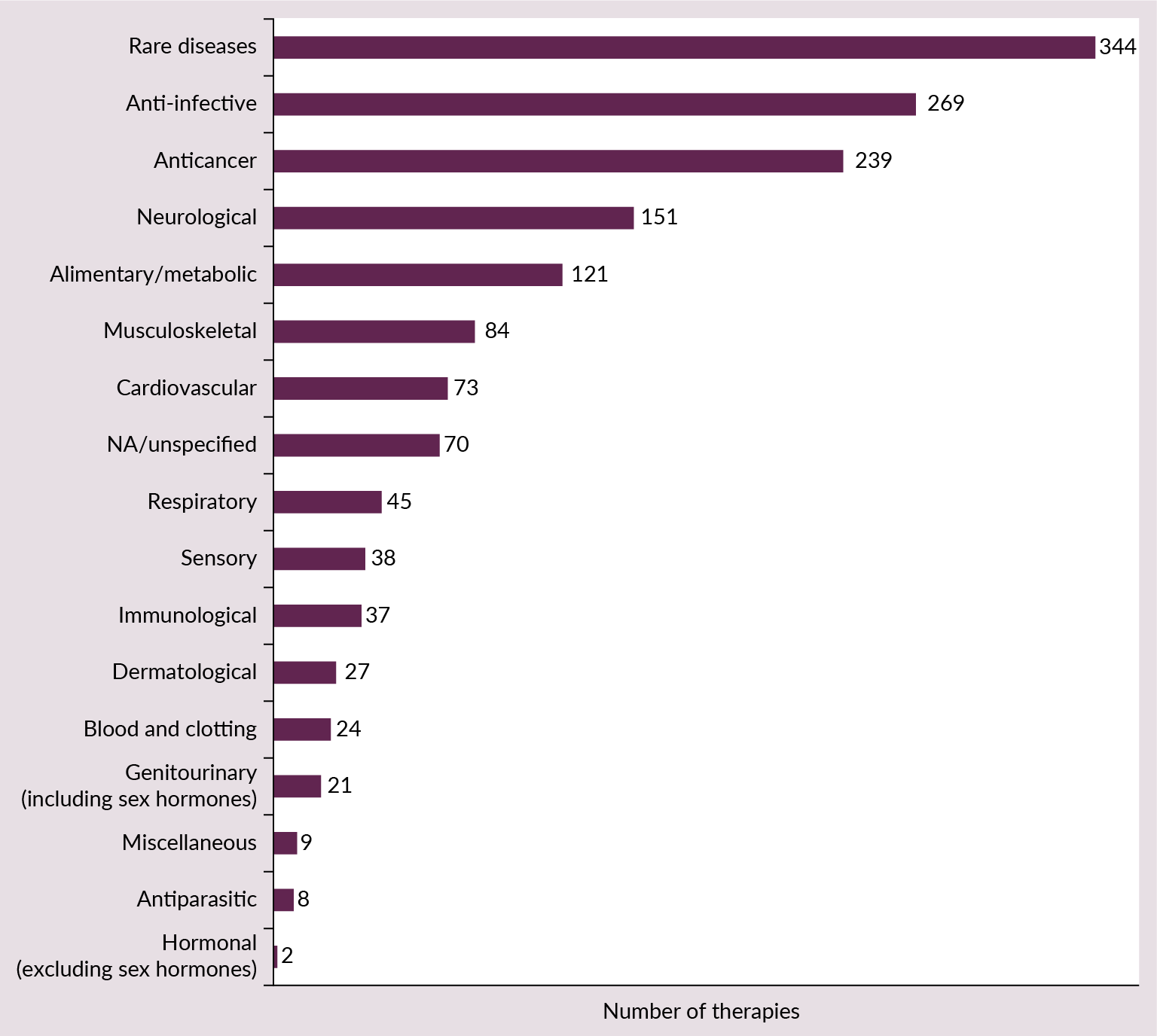 RNA therapeutics innovation landscape: key therapeutic areas are shown by the number of RNA therapies currently in the pipeline (from preclinical to pre-registration) [1]. ).
RNA therapeutics innovation landscape: key therapeutic areas are shown by the number of RNA therapies currently in the pipeline (from preclinical to pre-registration) [1]. ).
| Table 1. Overview of gene-based therapeutics. | ||
|---|---|---|
| RNA therapeutics | RNA aptamer | |
| ASO | ||
| RNAi | siRNA | |
| miRNA | ||
| mRNA | mRNA Tx | |
| mRNA vaccines | ||
| mRNA-based cell Tx | ||
| Gene editing | Nuclease | Meganuclease |
| TALEN | ||
| ZFN | ||
| CRISPR/Cas | CRISPR/Cas | |
| Base editing | ||
| Prime editing | ||
| Epigenetic editing | ||
Despite the promising number of therapies in development, the path from laboratory to clinic is fraught with challenges, particularly in translating preclinical findings to human outcomes. Of the RNA therapeutics that enter clinical trials, only a small percentage will successfully navigate all phases to reach market approval, underscoring the critical importance of accurate preclinical modeling in the successful advancement of these therapeutics.
Traditional animal models, while valuable, are often inadequate in predicting human responses due to species-specific differences in physiology, metabolism, and disease manifestation. This fundamental disconnect frequently leads to late-stage failures in clinical trials, leading to significant time and resource expenditures. Clinical failures can be attributed to a combination of reasons including a lack of clinical efficacy or toxicity (70–80%), poor drug properties (10–15%) or commercial reasons (10%) [3]Sun D, Gao W, Hu H, Zhou S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022; 12(7), 3049–3062.. For RNA therapeutics in particular, delivery of the drug to target organs/tissues and toxicity due to off-target binding are some of the most significant challenges [4]Ali Zaidi SS, Fatima F, Ali Zaidi SA, Zhou D, Deng W, Liu S. Engineering siRNA therapeutics: challenges and strategies. J. Nanobiotechnol. 2023; 21(1), 381..
In this article, we examine the use of humanized chimeric mouse models, specifically the PXB-mouse as a solution to improve preclinical predictions of the efficacy and safety of RNA therapeutics, with a particular focus on siRNAs due to their current prevalence. By addressing the limitations of traditional animal models, these advanced preclinical tools offer a more translatable platform for RNA therapeutic development, potentially reducing late-stage failures and accelerating the path to successful treatments.
Challenges with traditional animal models
The development of RNA therapeutics, particularly siRNAs, faces several hurdles when relying on traditional animal models, such as mice, which can lead to misleading preclinical results.
Key limitations of traditional models include:
- Species-specific differences in physiology and metabolism
- Poor prediction of human pharmacokinetics and pharmacodynamics
- Inadequate modelling of human-specific infections (such as viral hepatitis)
- Inability to directly target human genes in vivo during preclinical studies
Together, these factors contribute to a significant translational gap, where promising results observed in animal studies fail to materialize in human trials.
PXB-mouse: a humanized liver chimeric model
Humanized liver chimeric models, such as the PXB-mouse (described below), have been developed to address the translational challenges. Such models provide a more human-relevant environment for the preclinical testing of RNA therapeutics.
The PXB-mouse is created using transgenic mice that allow for the ablation of endogenous mouse hepatocytes and accept the engraftment of xenotransplanted human hepatocytes [5]Tateno C, Yoshizane Y, Saito N, et al. Near completely humanized liver in mice shows human-type metabolic responses to drugs. Am. J. Pathol. 2004; 165(3), 901-912. [6]Tateno C, Kawase Y, Tobita Y, et al. Generation of novel chimeric mice with humanized livers by using hemizygous cDNA-uPA/SCID mice. PLoS One 2015; 10(11), e0142145. [7]Sugahara G, Ishida Y, Sun J, Tateno C, Saito T. Art of making artificial liver: depicting human liver biology and diseases in mice. Semin. Liver Dis. 2020; 40(2), 189–212.. This process results in a mouse with up to 95% of its liver repopulated with functional human hepatocytes. The outcome is a chimeric model that combines the benefits of a small animal model with the biological relevance of human liver tissue.
Features of the PXB-mouse include:
- Normal human liver histology and function [8]
- Human-specific metabolism and excretion pathways [9–11]
- Expression of human genes, mRNA, and proteins [8,12]
- Human-like lipoprotein profiles [13]
- Production of human albumin and human-like biliary excretion [6,14,15]
- Permissive to infection with HBV and HDV [16,17]
However, it should be noted that while human chimeric models, such as PXB-mouse, contain human hepatocytes in the liver, they lack the human liver immune and stromal cells that would also play a role in disease responses in humans.
Given its unique characteristics, the PXB-mouse serves as an invaluable tool for studying responses to small molecule drugs, biologics, gene therapy delivery systems and RNA therapeutics. The presence of human hepatocytes allows for direct targeting of human genes in vivo at the preclinical stage, more relevant delivery to human hepatocytes, and simultaneous evaluation of toxicity and pharmacodynamics. Consequently, these models aid the early identification of potential efficacy and safety issues, leading to better-informed clinical trials.
Humanized liver models in action
There are many reasons for the failure of most RNA therapeutics, including off-target effects, delivery challenges, target engagement challenges and low therapeutic efficacy. Despite these obstacles, humanized liver mouse models have consistently proven effective in recapitulating human outcomes in RNA therapeutic development. Here we examine four examples from a wide range of published literature that showcase the value of humanized liver mouse models in this field.
Case study 1: Overcoming off-target effects and safety challenges: RNAi therapeutics for chronic hepatitis B
Chronic hepatitis B virus (cHBV) infection remains a significant global health challenge; the WHO estimated 254 million people were living with the condition in 2022 plus 1.2 million new infections each year [18]World Health Organization. Fact Sheet. Hepatitis B. Apr 9, 2024. . RNA interference (RNAi) therapeutics have shown promise in targeting cHBV, but safety concerns have hindered their development.
HBV is associated with the expression of various proteins including hepatitis B surface antigen (HBsAg). It is hypothesized that large quantities of HBsAg contribute to T- and B-cell dysfunction, impairing the host’s ability to eradicate the HBV infection. A potential treatment involves reducing HBsAg using RNA interference via siRNA. Since there are overlapping templates within the X region of the HBV genome, a single siRNA could selectively and effectively target all HBV transcripts [19]Gane E, Lim Y, Kim JB, et al. Evaluation of RNAi therapeutics VIR-2218 and ALN-HBV for chronic hepatitis B: Results from randomized clinical trials. J. Hepatol. 2023; 79(4), 924–932..
In a recent study, researchers used the PXB-mouse as a preclinical model to accurately predict the safety and tolerability of investigational RNAi therapeutics in healthy volunteers [19]Gane E, Lim Y, Kim JB, et al. Evaluation of RNAi therapeutics VIR-2218 and ALN-HBV for chronic hepatitis B: Results from randomized clinical trials. J. Hepatol. 2023; 79(4), 924–932.. The study compared two siRNAs that target all major HBV mRNA transcripts: ALN-HBV and VIR-2218. These siRNAs have the same sequences, except that VIR-2218 has been chemically modified via enhanced stabilization chemistry plus (ESC+) resulting in a single substitution of a glycol nucleic acid modification within the seed region. This modification was an attempt to minimize off-target effects.
PXB-mice (12–18 weeks of age) received subcutaneous injections of ALN-HBV or VIR-2218 at doses of 12, 36, or 100 mg per kg of animal weight. Blood analysis over seven weeks revealed markedly lower alanine aminotransferase (ALT) levels following administration of VIR-2218 compared to ALN-HBV, indicating reduced liver damage.
Importantly, these preclinical findings were supported by subsequent clinical studies in healthy volunteers. Here, the PXB-mouse model accurately predicted the improved safety profile of VIR-2218, demonstrating its value in assessing potential off-target effects and safety concerns early in the development process.
Case study 2: Overcoming delivery challenges: effective use of LNPs in PXB-mice
Effective delivery of RNAi therapeutics is as crucial as their sequence design (Figure 2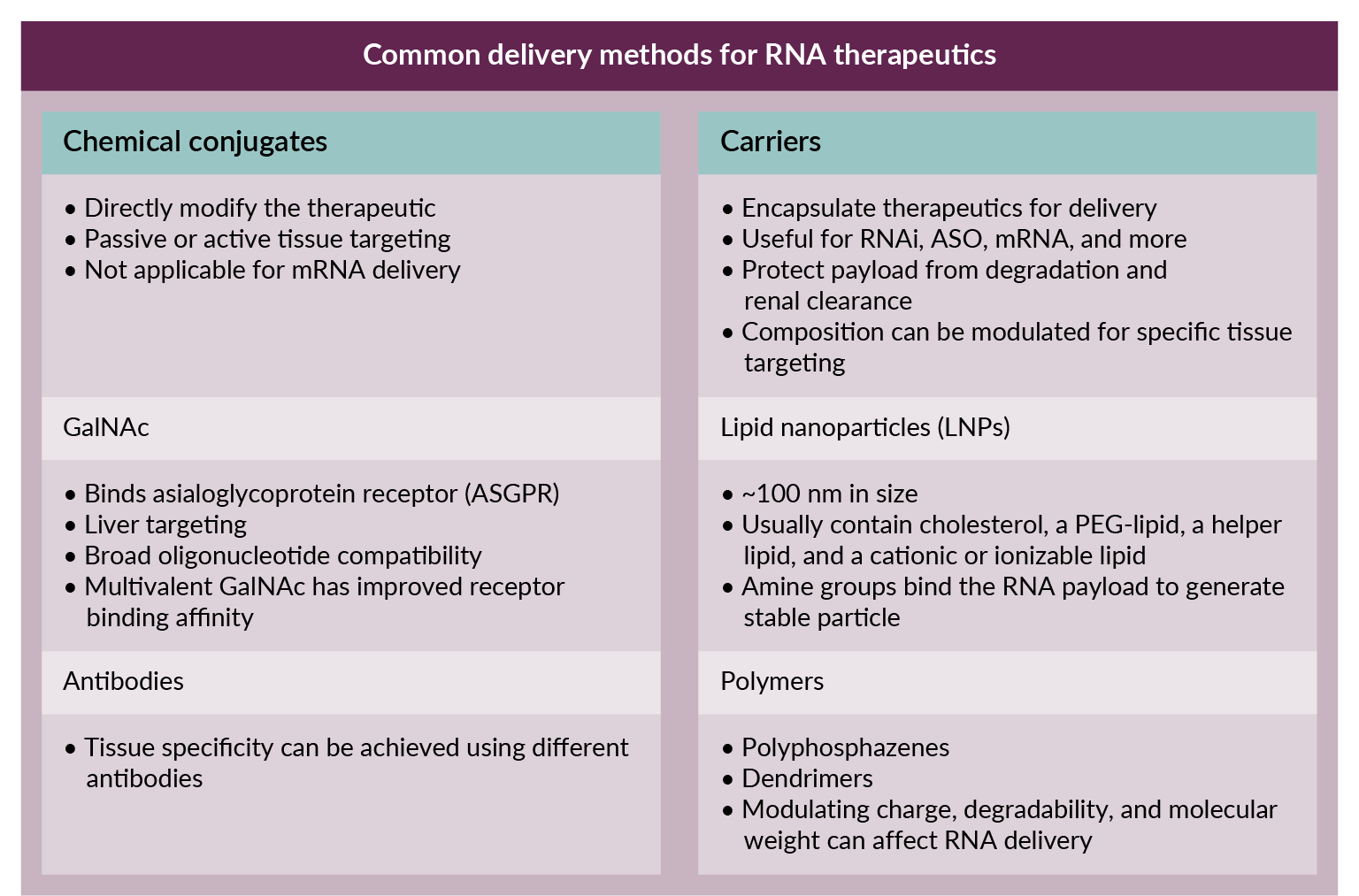 Overview of RNA therapeutic delivery methods.). Multiple options are available, but lipid nanoparticles (LNPs) have emerged as a popular choice for siRNA delivery due to their ability to target various tissues while protecting the siRNA from degradation. (Most recently, the spotlight was on LNPs as a key component of the COVID-19 vaccines [20]Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mat. 2021; 6, 1078–1094. [21]Lam K, Schreiner P, Leung A, et al. Optimizing lipid nanoparticles for delivery in primates. Adv. Mater. 2023; 35(26), e2211420.). Despite these advancements, issues in ensuring RNAi uptake by the correct tissue and achieving cross-species compatibility can complicate preclinical testing. This is where humanized liver mouse models offer a distinct advantage, helping to bridge these gaps by closely mimicking human responses to RNA therapeutics.
Overview of RNA therapeutic delivery methods.). Multiple options are available, but lipid nanoparticles (LNPs) have emerged as a popular choice for siRNA delivery due to their ability to target various tissues while protecting the siRNA from degradation. (Most recently, the spotlight was on LNPs as a key component of the COVID-19 vaccines [20]Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mat. 2021; 6, 1078–1094. [21]Lam K, Schreiner P, Leung A, et al. Optimizing lipid nanoparticles for delivery in primates. Adv. Mater. 2023; 35(26), e2211420.). Despite these advancements, issues in ensuring RNAi uptake by the correct tissue and achieving cross-species compatibility can complicate preclinical testing. This is where humanized liver mouse models offer a distinct advantage, helping to bridge these gaps by closely mimicking human responses to RNA therapeutics.
In a study by Okada et al., an LNP encapsulated siRNA was effectively used to target Dock11, a host factor regulating covalently closed circular DNA (cccDNA) formation by HBV in PXB-mice [22]Okada H, Sakamoto T, Nio K, et al. Lipid nanoparticle-encapsulated DOCK11-siRNA efficiently reduces hepatitis B virus cccDNA level in infected mice. Mol. Ther. Methods Clin. Dev. 2024; 32(3), 101289.. cccDNA forms during HBV replication and acts as a viral reservoir in cells. The persistence of cccDNA and the inability to effectively target it with therapeutics is a key reason that a cure for HBV remains elusive.
In PXB-mice, the LNP-encapsulated siRNA targeting DOCK11 showed highly effective knockdown of human DOCK11 in PXB-mice and, importantly, a clear reduction in cccDNA levels. The study employed the same LNP formulation as that used in the FDA-approved drug Onpattro®, highlighting the cross-compatibility of human-tested LNPs in the PXB-mouse.
Another example of the effective use of LNPs in PXB-mice to predict human outcomes comes from a hepatitis delta virus (HDV) study [17]Ye X, Tateno C, Thi EP, et al. Hepatitis B virus therapeutic agent ARB-1740 has inhibitory effect on hepatitis delta virus in a new dually-infected humanized mouse model. ACS Infect Dis. 2019; 5(5), 738–749.. HDV infects an estimated 10−20 million people globally and is associated with severe fulminant hepatitis, which often leads to cirrhosis and an increased risk of hepatocellular carcinoma. Despite the severity of the disease, there is an unmet clinical need for effective treatments.
HDV infection requires the presence of HBsAg; HDV can either establish itself as a superinfection in individuals already carrying HBV or through a simultaneous coinfection when a person is exposed to both HBV and HDV at the same time.
In this study, researchers used humanized mice dually infected with both HBV and HDV to evaluate the effectiveness of HBV-targeting siRNA therapy in controlling HDV infection, comparing it to a direct anti-HDV siRNA approach.
The results revealed that in vivo treatment with an anti-HBV RNAi agent successfully reduced both HBV and HDV viremia, showing the potential of this approach in managing HDV infection.
Specifically, treatment with ARB-1740, delivered via LNP technology, resulted in a 2.3 log10 reduction in HBV viremia and a 2.6 log10 decrease in serum HBsAg levels, which led to a subsequent 1.6 log10 reduction in HDV viremia. In contrast, HDV-targeting siRNA effectively inhibited HDV in both the blood and liver compartments without impacting HBV. Additionally, PEGylated interferon-alpha reduced HBV viremia by 2.0 log10 but did not affect HDV viremia under the conditions of this study. These findings demonstrate the inhibitory effect of ARB-1740 on HDV, supporting its potential as a therapeutic option.
Note that, as anticipated by the investigators, the human chimeric mouse model showed no overt signs of liver damage (including cirrhosis or hepatocellular carcinoma) in either of the coinfection or superinfection studies described, mostly likely due to the lack of an adaptive immune response in this system.
Overall, these studies emphasize the value of the humanized liver mouse model in generating translationally relevant results, which can guide the selection of effective siRNA delivery methods.
Case study 3: Target engagement and therapeutic efficacy: hepatocyte-targeted siTAZ therapy in MASH
Metabolic dysfunction-associated steatohepatitis (MASH), formerly known as non-alcoholic steatohepatitis (NASH), is becoming the most common cause of liver disease. To date, therapies that have shown promise in mouse MASH models have not translated well to humans, underscoring the need for more predictive preclinical models [23]Xie Z, Li Y, Cheng L, et al. Potential therapeutic strategies for MASH: from preclinical to clinical development. Life Metab. 2024; 5(5), loae029. [24]Younossi ZM, Ratziu V, Loomba R, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled Phase 3 trial. Lancet 2019; 394(10215), 2184–2196..
It has been shown that MASH can be established in PXB-mice by feeding them a high fat diet [25]Kisoh K, Sugahara G, Ogawa Y, et al. Estimating drug efficacy with a diet-induced NASH model in chimeric mice with humanized livers. Biomedicines 2021; 9(11), 1647.. PXB-mice fed these diets recapitulate the key features of human metabolic dysfunction-associated fatty liver disease (MAFLD)/MASH including hepatocyte ballooning, inflammation, and importantly, fibrosis.
Researchers used MASH diet-fed PXB-mice to test GalNAc-siTaz, an siRNA targeting the gene for TAZ, a transcriptional regulator [26]Wang X, Moore MP, Shi H, et al. Hepatocyte-targeted siTAZ therapy lowers liver fibrosis in NASH diet-fed chimeric mice with hepatocyte-humanized livers. Mol. Ther. Methods Clin. Dev. 2023; 31, 101165.. The researchers chose this model reasoning that ‘a mouse NASH model whose livers are populated with human hepatocytes would be particularly valuable in testing hepatocyte-targeted siRNA therapies’.
The PXB-mice were fed a high-fat, choline-deficient, L-amino acid-defined diet for 6 weeks to induce MASH. This was followed by 6-weekly injections of GalNAc-siTAZ or a control siRNA (GalNAc-control) while maintaining the MASH diet.
The results were promising. GalNAc-siTAZ lowered human hepatic TAZ and IHH (Indian hedgehog) a TAZ target that promotes MASH fibrosis. In addition, treatment with GalNAc-siTAZ decreased liver inflammation, hepatocellular injury, hepatic fibrosis, and profibrogenic mediator expression compared to the control. These effects indicated that GalNAc-siTAZ decreased the progression of MASH in mice reconstituted with human hepatocytes.
This study demonstrates the value of humanized liver models in assessing both target engagement and therapeutic efficacy for complex metabolic liver diseases like MASH.
Case study 4: Target engagement and therapeutic efficacy: STP125G for hypertriglyceridemia
Hypertriglyceridemia, characterized by elevated triglyceride levels, is associated with an increased risk of cardiovascular diseases. In cases of severe hypertriglyceridemia (sHTG), where triglyceride levels exceed 1,000 mg/dL, the risk of developing acute pancreatitis is 5–10 times greater than in the general population.
STP125G, an siRNA therapeutic targeting apolipoprotein C3 (ApoC3), developed by Sirnanomics, was tested in PXB-mice to demonstrate its efficacy in reducing triglyceride levels [27]Samarsky D. GalAhead™ Platform & Programs. Sirnaomics. . ApoC3 is a key player in triglyceride metabolism and has recently been recognized as a factor influencing cardiovascular, metabolic, and neurological disease risk.
The study in PXB-mice showed a single dose of STP125G resulted in high-efficiency, durable knockdown of ApoC3, and significant reductions in both mRNA and protein levels of ApoC3 were observed up to 6 weeks post-treatment. Corresponding reductions in triglycerides and cholesterol were observed, returning to control levels by week 8.
The results in the humanized liver model provided strong support for ApoC3-targetting siRNA as a therapeutic approach in hypertriglyceridemia management, paving the way for clinical development.
FDA guidance on choosing animal models for cell and gene therapies
The FDA’s Center for Biological Evaluation and Research (CBER) has provided guidance on selecting relevant animal models for cell and gene therapy (CGT) assessments, including RNA therapeutics [28]US Food and Drug Administration. Guidance for Industry. Preclinical Assessment of Investigational Cellular and Gene Therapy Products. Nov 2013. . This guidance emphasizes key considerations that align with the advantages of humanized chimeric models (Table 2).
| Table 2. Alignment of humanized mouse models with CBER guidance. | |
|---|---|
| CBER recommendation | Benefits of humanized mouse models |
| Select animal species that closely reflect the biological response expected in humans | Direct targeting of human genes is possible in the humanized liver of these mouse models |
| Consider physiological and anatomical comparability to humans | Liver expresses human metabolic pathways and transporters in physiologically relevant zonation patterns, and they also have humanized lipoprotein profiles [9,12,13] |
| Assess permissiveness/susceptibility to infection by, and replication of, viral vectors or microbial vectors for gene therapy | Humanized liver mice are permissive to AAV and adenovirus vectors, commonly used in gene therapies [29–31] |
| Evaluate immune tolerance to human cell therapy products or human transgenes expressed by gene therapy products | |
| Ensure feasibility of using the planned clinical delivery system/procedure | Amenable to therapeutically relevant delivery methods, including GalNAc-conjugated RNAs, LNPs and adeno-associated viruses (AAVs), and provide a system to directly test human-targeting RNAi therapeutics in vivo |
Humanized liver mice fit many of these criteria as their liver expresses human metabolic pathways and transporters in physiologically relevant zonation patterns, and they also have humanized lipoprotein profiles [9]Kakuni M, Yamasaki C, Tachibana A, Yoshizane Y, Ishida Y, Tateno C. Chimeric mice with humanized livers: a unique tool for in vivo and in vitro enzyme induction studies. Int. J. Mol. Sci. 2013; 15(1), 58–74. [12]Ohtsuki S, Kawakami H, Inoue T, et al.Validation of uPA/SCID mouse with humanized liver as a human liver model: protein quantification of transporters, cytochromes P450, and UDP-glucuronosyltransferases by LC-MS/MS. Drug Metab. Dispos. 2014; 42(6), 1039–1043. [13]Papazyan R, Liu X, Liu J, et al. FXR activation by obeticholic acid or nonsteroidal agonists induces a human-like lipoprotein cholesterol change in mice with humanized chimeric liver. J. Lipid Res. 2018; 59(6), 982–993.. Moreover, these models are amenable to therapeutically relevant delivery methods, including LNPs and adeno-associated viruses (AAVs), and provide a system to directly test human-targeting RNAi therapeutics in vivo.
Sponsors are encouraged to submit detailed species assessments as part of the preclinical section of the IND. To support these assessments, there are more than 300 publications featuring the PXB-mouse over the last 20 years, meaning it has been thoroughly characterized for a variety of applications including CGT, with a deep body of work to draw from when interpreting new data. Cumulatively, these studies show that the PXB-mouse is a valuable model for viral hepatitis and predicting human-specific response to therapeutics. Some limitations have also been identified, including dysregulation of some pathways where there is a mismatch in human and mouse signaling, for example, human growth factor (hGF) signaling [29]Ito M, Takino N, Nomura T, Kan A, Muramatsu S. Engineered adeno‑associated virus 3 vector with reduced reactivity to serum antibodies. Nat. Sci. Rep. 2021; 11, 9322.. In addition, the PK profiles of therapeutic compounds that show very high clearance in mice may not be accurate in humanized liver mouse models [10]Bateman TJ, Reddy VG, Kakuni M, Morikawa Y, Kumar S. Application of chimeric mice with humanized liver for study of human-specific drug metabolism. Drug Metab. Dispos. 2014; 42(6), 1055–1065..
CBER also encourages using disease/injury animal models in preclinical studies for CGT products. Due to the unique features of CGT products—such as prolonged effects, persistence in vivo, complex mechanisms of action, and invasive routes of administration—using disease models rather than healthy animals is preferred for assessing activity and safety. As shown above, PXB-mouse can be used as a MASH model and is the gold standard for viral hepatitis. While the PXB-mouse is usually produced using healthy donor hepatocytes, it’s also possible to generate disease models, such ornithine transcarbamylase deficiency (OTCD) mice [30]Zhang S, Bastille A, Gordo S, et al. Novel AAV-mediated genome editing therapy improves health and survival in a mouse model of methylmalonic acidemia. PLOS ONE 2022; 17(9), e0274774..
It’s important to note that no single model can perfectly predict the efficacy and safety of an investigational CGT product in humans. However, the PXB-mouse can be a key part of a comprehensive program of efficacy and safety testing, allowing for greater confidence when entering the clinic with a new therapeutic.
Translation insight
The development of RNA therapeutics, particularly siRNAs, represents a promising frontier in modern medicine. Yet, the path from preclinical studies to successful clinical outcomes is fraught with challenges, many of which stem from the limitations of traditional animal models.
Humanized liver chimeric models, such as the PXB-mouse, offer a powerful solution to these challenges. By providing a more human-relevant environment for preclinical testing, these models enable direct targeting of human genes in vivo and more accurate assessment of drug metabolism and pharmacokinetics. As a result, you obtain a better prediction of efficacy and safety in humans and can evaluate therapeutics against human-specific pathogens.
References
1. American Society of Gene and Cell Therapy and Citeline. Gene, Cell, and RNA Therapy Landscape Report. 2024. Link
2. Lutzmayer S, Wright A. IQVIA pipeline intelligence. IQVIA Nov 27, 2023. Link
3. Sun D, Gao W, Hu H, Zhou S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022; 12(7), 3049–3062. Crossref
4. Ali Zaidi SS, Fatima F, Ali Zaidi SA, Zhou D, Deng W, Liu S. Engineering siRNA therapeutics: challenges and strategies. J. Nanobiotechnol. 2023; 21(1), 381. Crossref
5. Tateno C, Yoshizane Y, Saito N, et al. Near completely humanized liver in mice shows human-type metabolic responses to drugs. Am. J. Pathol. 2004; 165(3), 901-912. Crossref
6. Tateno C, Kawase Y, Tobita Y, et al. Generation of novel chimeric mice with humanized livers by using hemizygous cDNA-uPA/SCID mice. PLoS One 2015; 10(11), e0142145. Crossref
7. Sugahara G, Ishida Y, Sun J, Tateno C, Saito T. Art of making artificial liver: depicting human liver biology and diseases in mice. Semin. Liver Dis. 2020; 40(2), 189–212. Crossref
8. Tateno C, Miya F, Wake K, et al. Morphological and microarray analyses of human hepatocytes from xenogeneic host livers. Lab. Invest. 2013; 93(1), 54–71. Crossref
9. Kakuni M, Yamasaki C, Tachibana A, Yoshizane Y, Ishida Y, Tateno C. Chimeric mice with humanized livers: a unique tool for in vivo and in vitro enzyme induction studies. Int. J. Mol. Sci. 2013; 15(1), 58–74. Crossref
10. Bateman TJ, Reddy VG, Kakuni M, Morikawa Y, Kumar S. Application of chimeric mice with humanized liver for study of human-specific drug metabolism. Drug Metab. Dispos. 2014; 42(6), 1055–1065. Crossref
11. Sanoh S, Naritomi Y, Fujimoto M, et al. Predictability of plasma concentration-time curves in humans using single-species allometric scaling of chimeric mice with humanized liver. Xenobiotica 2015 ;45(7), 605–614. Crossref
12. Ohtsuki S, Kawakami H, Inoue T, et al.Validation of uPA/SCID mouse with humanized liver as a human liver model: protein quantification of transporters, cytochromes P450, and UDP-glucuronosyltransferases by LC-MS/MS. Drug Metab. Dispos. 2014; 42(6), 1039–1043. Crossref
13. Papazyan R, Liu X, Liu J, et al. FXR activation by obeticholic acid or nonsteroidal agonists induces a human-like lipoprotein cholesterol change in mice with humanized chimeric liver. J. Lipid Res. 2018; 59(6), 982–993. Crossref
14. Fujino C, Sanoh S, Tamura Y, et al. Changes in bile acid concentrations in chimeric mice transplanted with different replacement indexes of human hepatocytes. BPB Reports 2019; 2, 29–34. Crossref
15. Sanoh S, Tamura Y, Fujino C, et al. Changes in bile acid concentrations after administration of ketoconazole or rifampicin to chimeric mice with humanized liver. Biol Pharm Bull. 2019; 42(8), 1366–1375. Crossref
16. Ishida Y, Chung TL, Imamura M, et al. Acute hepatitis B virus infection in humanized chimeric mice has multiphasic viral kinetics. Hepatology 2018; 68(2), 473–484. Crossref
17. Ye X, Tateno C, Thi EP, et al. Hepatitis B virus therapeutic agent ARB-1740 has inhibitory effect on hepatitis delta virus in a new dually-infected humanized mouse model. ACS Infect Dis. 2019; 5(5), 738–749. Crossref
18. World Health Organization. Fact Sheet. Hepatitis B. Apr 9, 2024. Link
19. Gane E, Lim Y, Kim JB, et al. Evaluation of RNAi therapeutics VIR-2218 and ALN-HBV for chronic hepatitis B: Results from randomized clinical trials. J. Hepatol. 2023; 79(4), 924–932. Crossref
20. Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mat. 2021; 6, 1078–1094. Crossref
21. Lam K, Schreiner P, Leung A, et al. Optimizing lipid nanoparticles for delivery in primates. Adv. Mater. 2023; 35(26), e2211420. Crossref
22. Okada H, Sakamoto T, Nio K, et al. Lipid nanoparticle-encapsulated DOCK11-siRNA efficiently reduces hepatitis B virus cccDNA level in infected mice. Mol. Ther. Methods Clin. Dev. 2024; 32(3), 101289. Crossref
23. Xie Z, Li Y, Cheng L, et al. Potential therapeutic strategies for MASH: from preclinical to clinical development. Life Metab. 2024; 5(5), loae029. Crossref
24. Younossi ZM, Ratziu V, Loomba R, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled Phase 3 trial. Lancet 2019; 394(10215), 2184–2196. Crossref
25. Kisoh K, Sugahara G, Ogawa Y, et al. Estimating drug efficacy with a diet-induced NASH model in chimeric mice with humanized livers. Biomedicines 2021; 9(11), 1647. Crossref
26. Wang X, Moore MP, Shi H, et al. Hepatocyte-targeted siTAZ therapy lowers liver fibrosis in NASH diet-fed chimeric mice with hepatocyte-humanized livers. Mol. Ther. Methods Clin. Dev. 2023; 31, 101165. Crossref
27. Samarsky D. GalAhead™ Platform & Programs. Sirnaomics. Link
28. US Food and Drug Administration. Guidance for Industry. Preclinical Assessment of Investigational Cellular and Gene Therapy Products. Nov 2013. Link
29. Ito M, Takino N, Nomura T, Kan A, Muramatsu S. Engineered adeno‑associated virus 3 vector with reduced reactivity to serum antibodies. Nat. Sci. Rep. 2021; 11, 9322. Crossref
30. Zhang S, Bastille A, Gordo S, et al. Novel AAV-mediated genome editing therapy improves health and survival in a mouse model of methylmalonic acidemia. PLOS ONE 2022; 17(9), e0274774. Crossref
31. Kubo S, Kataoka M, Tateno C, et al. In vivo stable transduction of humanized liver tissue in chimeric mice via high-capacity adenovirus–
lentivirus hybrid vector. Hum. Gene Ther. 2021; 21, 40–50. Crossref
32. Tateno C, Kataoka M, Utoh R, et al. Growth hormone-dependent pathogenesis of human hepatic steatosis in a novel mouse model bearing a human hepatocyte-repopulated liver. Endocrinology 2011; 152(4), 1479–1491. Crossref
33. Sugahara G, Yamasaki C, Yanagi A, et al. Humanized liver mouse model with transplanted human hepatocytes from patients with ornithine transcarbamylase deficiency. J. Inherit. Metab. Dis. 2020; 44(3), 618–628. Crossref
Affiliations
Matthew Baginski
Executive Vice President,
Strategy and Business Development,
PhoenixBio USA,
New York, NY, USA
Sara Donnelly PhD
Director, Research Planning & Business Development,
PhoenixBio USA,
New York, NY, USA
Authorship & Conflict of Interest
Contributions: The named author takes responsibility for the integrity of the work as a whole, and has given their approval for this version to be published.
Acknowledgements: The authors would like to thank Yoshinari Miyata, Chief Innovation Officer, PhoenixBio, for contributing Figure 1.
Disclosure and potential conflicts of interest: The authors are employees of PhoenixBio USA.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & Copyright Information
Copyright: Published by Nucleic Acid Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2024 PhoenixBio USA. Published by Nucleic Acid Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Oct 3, 2024; Revised manuscript received: Nov 13, 2024; Publication date: Nov 21, 2024.

