Chemical modifications for therapeutic aptamers
Nucleic Acid Insights 2025; 2(2), 43–59
DOI: 10.18609/nuc.2025.010
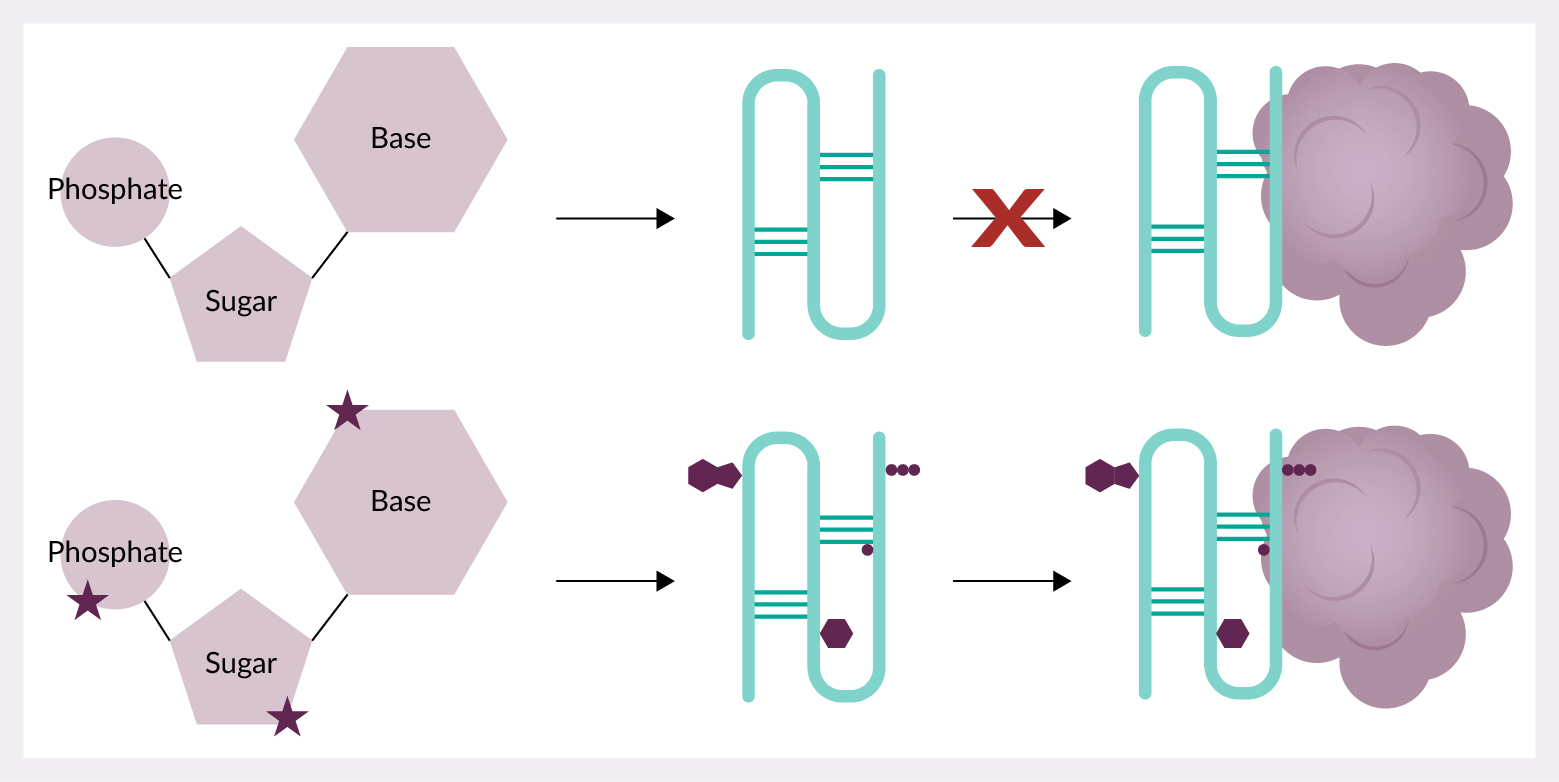
Oligonucleotide aptamers, with their ability to bind diverse biological targets, may be promising successors to therapeutic antibodies in clinical applications due to their reduced production times, ease of synthesis and in vitro discovery protocol which does not require animal use or target immunogenicity. However, progress has stalled due to challenges of nuclease degradation, renal filtration, and binding thermodynamics. Some aptamers have overcome these hurdles, reaching FDA approval, and chemical modifications have played a pivotal role in their success. Chemical modifications give improvements to the binding affinity and selectivity, stability, and pharmacokinetics of aptamers, over natural nucleotides. However, changes made to monomers can also alter the overall 3D structure, having significant impact on an aptamer’s effect. Identifying the best set of modifications for each therapeutic need will play a key role in the future of therapeutic aptamers.
 |
