Unlocking mRNA-LNP potential using a novel lipid library combined with innovative analytical methods
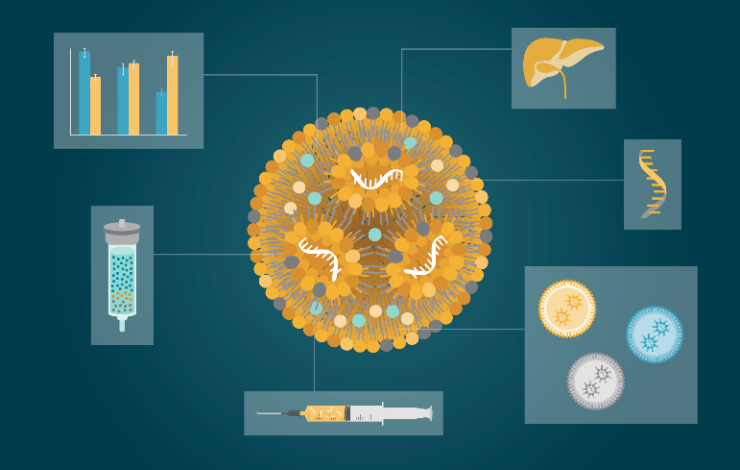
Lipid nanoparticles (LNPs) have emerged as a versatile platform for RNA delivery, finding extensive use in both in vivo and ex vivo applications such as prophylactic and therapeutic vaccines, gene editing, protein replacement therapies and genetically modified cell therapies. Despite their numerous advantages, LNPs still exhibit some limitations, notably their tendency to accumulate in the liver, causing loss of efficiency and off-target effects. Addressing this challenge is a top priority within the field, as it holds the key to unlocking the full therapeutic potential of these innovative treatments. In addition, post-formulation, LNPs require downstream processing and monitoring of Critical Quality Attributes (CQAs). Accurate and reliable analytical methods are therefore essential to ensure the quality, efficacy, and safety of LNPs in various applications.
This webinar will showcase the potential of an innovative library of imidazolium-based lipids in the formulation of LNPs, highlighting their potential to fully replace or complement ionizable lipids. Comprehensive characterization data of the LNPs produced will be presented, and the value of screening the library to identify the best suited lipid formulations for specific therapeutic objectives will be demonstrated.
Further, liquid chromatrography will be discussed as an invaluable analytical tool which can be utilized in this space. Using liquid chromatrography, researchers and formulation scientists can gain critical insights into the encapsulation efficiency, lipid composition and quantification, load integrity and size of LNPs loaded with nucleic acids. These analytical methods contribute to the development of robust and efficient LNP drug products, ultimately enhancing the therapeutic outcomes of nucleic acid-based therapies.
Attend the webinar to gain insight on:
- Optimizing LNP formulations and enable their comprehensive analysis
- Benefits of innovative lipids on the properties of LNPs, as a total replacement for the ionizable lipid or as a complement
- New solutions for RNA therapeutics developers seeking to cure extrahepatic diseases or avoid off-target hepatic effects
- The value of utilizing reverse-phase chromatography for LNP analysis
- 2D chromatography for direct analysis of LNP formulation, with no sample pretreatment
You might also like
Beyond the liver: a novel lipid library opening new horizons for LNP developers

Pure and simple: understanding LNP analytics for better mRNA-based drugs

Elevate LNP optimization with Sunshine and Stunner



