Discovery to delivery in 100 days: RNA therapeutics & their role in future pandemic preparedness
Vaccine Insights 2022; 1(4), 267–277
DOI: 10.18609/vac.2022.38
Recently, the Coalition Epidemic Preparedness Innovations (CEPI) announced their ambition to develop vaccines against emerging diseases in 100 days. mRNA vaccines are a manufacturing modality that is suited to meet the 100-day strategy. The technology offers great benefits and potential for infectious diseases and personalized medicines due to the advantages in flexibility, cost, and speed of development, but there are still challenges to overcome to fully realize the potential. How can manufacturers prepare for rapid response?
Covid-19 changed the vaccine manufacturing landscape
The Covid-19 pandemic shone an intense spotlight on the vaccine industry, placing an unprecedented demand on biopharma manufacturers to develop and produce a vaccine in a severely shortened timeframe. Fortunately, what emerged were mRNA vaccines, which proved the potential of this type of nucleic acid–based therapeutic to be developed under a fast timeline while also showing high efficacy rates.
Following on from the lessons learnt during the pandemic, the Coalition for Epidemic Preparedness and Innovations (CEPI), hosted the Global Pandemic Preparedness Summit in collaboration with the UK Government. One of the key questions asked was “What if it took 100 days to make a safe and effective vaccine against any virus?” Before the Covid-19 pandemic, a vaccine could take up to 10 years to develop, but this was condensed to just 326 days. Producing a vaccine in 100 days could save lives, decrease economic damage, and possibly even prevent outbreaks from becoming pandemics. It’s an ambitious goal, but CEPI believe this is possible by tightening and shortening timelines at each stage [1]CEPI. 100 Days. CEPI 2022. (Accessed July 2022)..
Partnerships and collaborations were key to developing mRNA vaccines in a short time, and they will continue to be essential for the industry to achieve the 100-day timeline. While the coronavirus pandemic was a unique situation, it led to increased investment from governments, bilateral and multilateral donors, philanthropic organizations, development banks and private sector investors, into the vaccine industry and nucleic acid–based therapeutics overall [2]Cornish L. Who’s funding the COVID-19 response and what are the priorities? Devex (Jul 21 2021) (Accessed September 2022).. With proven potential to treat diseases in vivo and offer long-lasting effects, nucleic acid–based therapies will continue to spark biopharma’s creativity to develop more molecules with new functions to improve treatments and patient outcomes.
Advances in nucleic acid-based therapeutics
There have been extraordinary leaps in advancing development of many types of therapeutics within the last decade, in part due to advances in genomics, such as in bioinformatics and sequencing. This includes the extraordinary achievement of mapping the human genome, unlocking molecular pathways important in disease [3]NIH. The Human Genome Project. NIH. (Accessed September 2022)..
Different modalities can be used to produce different types of therapeutics, for example, by using viral vectors, DNA, mRNA, and proteins. Different modalities will have different manufacturing challenges as well as different advantages and disadvantages, depending on what is needed by the manufacturer to produce the therapeutic of choice.
Viral vector systems are the traditional, well-established method for producing vaccines, with proven efficacy through clinical trials. There are many successful candidates in place and manufacturers have developed low-cost facilities around the technology. However, it is slower compared to other methods and requires the use of animal cells. It is generally suitable for mid- to large-batch scale. Protein vaccines also use the well-established methods of the growth of living organisms but can be relatively complex to manufacture.
Because of the disadvantages to using traditional methods, there is high demand for alternative vaccines with clinical efficacy, high design flexibility, and fast manufacturing timelines. Developing new nucleic acid–based therapeutics is a research area where this demand could be met.
“Therapy with nucleic acids either uses unmodified DNA or RNA or closely related compounds. From both a development and regulatory perspective, they fall somewhere between small molecules and biologics. Several of these compounds are in clinical development and many have received regulatory approval for human use”
- Sridharan and Gogtay, 2016 [4]Sridharan K, Gogtay NJ. Therapeutic nucleic acids: current clinical status. Br. J. Clin. Pharmacol. 2016; 82(3), 659–672.
Nucleic acid–based therapeutics have particularly benefited from increased investment, collaboration, and partnerships, from the Covid-19 pandemic. Foreign Direct Investment (FDI) grew by 52% in 2021 according to GlobalData [5]Karadima S, Vaidya M. Foreign investments amplify the nucleic acid therapeutics field. Pharmaceutical Technology (Aug 25 2022). (Accessed September 2022). with investments made to organizations providing services or products related to genomics, DNA and RNA sequencing and genetic engineering. Some examples of this increased activity are shown below:
- Merck announced a collaboration agreement with Orna Therapeutics a biotechnology company pioneering a new investigational class of engineered circular RNA (oRNA) therapies [6];
- Eli Lilly have invested $ 700 million to create the Lilly Institute for Genetic Medicine, following their acquisition of Prevail Therapeutics, a gene therapy pioneer and investment into MiNA Therapeutics Ltd, a pioneer in RNA activation therapeutics [7,8];
- EtheRNA, a developer of mRNA therapeutics, has seen millions invested including from companies like Novalis [9];
- Arcturus, an mRNA medicines company, received $63.2 million from the US government to support development of saRNA vaccines [10].
With several start-ups now working in the early stage of next-generation RNA technologies, we should expect this interest to continue [11]Bell J. Next-generation RNA technologies: making longer-lasting drugs with a broader reach. BIOPHARMADIVE * Accessed September 2022. (Aug 23 2022). (Accessed September 2022)..
Nucleic acid–based therapeutics can be created using several sources. They include:
- DNA plasmids–small circular DNA molecule found in bacteria and other microscopic organisms that ranges in size from 4000–15000 base pairs;
- Protein-encoding mRNA–longer strand of mRNA, ranging from 100–20000 nucleotides, that is generally defined by the coding sequence it contains;
- Non-coding mRNA–shorter sequence of mRNA, ranging from 10–150 nucleotides, that does not contain a coding sequence (i.e., it is not translated into a protein)
The potential of RNA-based therapies
There are different types of RNA, each with a unique function. A useful distinction is to broadly classify RNA molecules into coding RNA (e.g., mRNA) or non-coding RNA (ncRNA) (Figure 1![RNA molecules classified into coding RNA (mRNA), where they carry the code for protein synthesis or non-coding. saRNA, circRNA and taRNA are shown here as having coding potential. ncRNAs do not undergo translation and there is a size different between the larger coding RNAs to the smaller ncRNAs. Examples of small ncRNAs include ribosomal RNA (rRNA), transfer RNA (tRNA), small nuclear RNAs (snRNA), piwi-interacting RNAs (piRNAs), micro RNAs (miRNA) and silencing RNA (siRNA). Some small ncRNAs are classed as having a regulatory effect and are involved in RNA silencing. miRNA modulates physiological and developmental gene expression. siRNA medicates sequence-specific cleavage of nascent mRNAs. piRNA may protect the germline from genome invaders [12]. Long ncRNAs (IncRNAs) are widely expressed and have key roles in gene regulation [13]. Categorization has been shown here based on action, but this is not exhaustive. mRNA can be in-vitro transcribed (IVT) for therapies [14] and for small ncRNA, oligo synthesis is an option [15]. RNA molecules classified into coding RNA (mRNA), where they carry the code for protein synthesis or non-coding. saRNA, circRNA and taRNA are shown here as having coding potential. ncRNAs do not undergo translation and there is a size different between the larger coding RNAs to the smaller ncRNAs. Examples of small ncRNAs include ribosomal RNA (rRNA), transfer RNA (tRNA), small nuclear RNAs (snRNA), piwi-interacting RNAs (piRNAs), micro RNAs (miRNA) and silencing RNA (siRNA). Some small ncRNAs are classed as having a regulatory effect and are involved in RNA silencing. miRNA modulates physiological and developmental gene expression. siRNA medicates sequence-specific cleavage of nascent mRNAs. piRNA may protect the germline from genome invaders [12]. Long ncRNAs (IncRNAs) are widely expressed and have key roles in gene regulation [13]. Categorization has been shown here based on action, but this is not exhaustive. mRNA can be in-vitro transcribed (IVT) for therapies [14] and for small ncRNA, oligo synthesis is an option [15].](https://cdn.insights.bio/uploads/Figure/C_CYT_204F1.png) Different types of coding mRNA and ncRNA molecules.RNA molecules classified into coding RNA (mRNA), where they carry the code for protein synthesis or non-coding. saRNA, circRNA and taRNA are shown here as having coding potential. ncRNAs do not undergo translation and there is a size different between the larger coding RNAs to the smaller ncRNAs. Examples of small ncRNAs include ribosomal RNA (rRNA), transfer RNA (tRNA), small nuclear RNAs (snRNA), piwi-interacting RNAs (piRNAs), micro RNAs (miRNA) and silencing RNA (siRNA). Some small ncRNAs are classed as having a regulatory effect and are involved in RNA silencing. miRNA modulates physiological and developmental gene expression. siRNA medicates sequence-specific cleavage of nascent mRNAs. piRNA may protect the germline from genome invaders [12]. Long ncRNAs (IncRNAs) are widely expressed and have key roles in gene regulation [13]. Categorization has been shown here based on action, but this is not exhaustive. mRNA can be in-vitro transcribed (IVT) for therapies [14] and for small ncRNA, oligo synthesis is an option [15].). Self-amplifying RNA (saRNA), circular RNA (circRNA), and trans-amplifying RNA (taRNA) have been shown to have coding potential, but their functionality is largely uncharacterized. Less than 2% of the human genome sequences encode for proteins.
Different types of coding mRNA and ncRNA molecules.RNA molecules classified into coding RNA (mRNA), where they carry the code for protein synthesis or non-coding. saRNA, circRNA and taRNA are shown here as having coding potential. ncRNAs do not undergo translation and there is a size different between the larger coding RNAs to the smaller ncRNAs. Examples of small ncRNAs include ribosomal RNA (rRNA), transfer RNA (tRNA), small nuclear RNAs (snRNA), piwi-interacting RNAs (piRNAs), micro RNAs (miRNA) and silencing RNA (siRNA). Some small ncRNAs are classed as having a regulatory effect and are involved in RNA silencing. miRNA modulates physiological and developmental gene expression. siRNA medicates sequence-specific cleavage of nascent mRNAs. piRNA may protect the germline from genome invaders [12]. Long ncRNAs (IncRNAs) are widely expressed and have key roles in gene regulation [13]. Categorization has been shown here based on action, but this is not exhaustive. mRNA can be in-vitro transcribed (IVT) for therapies [14] and for small ncRNA, oligo synthesis is an option [15].). Self-amplifying RNA (saRNA), circular RNA (circRNA), and trans-amplifying RNA (taRNA) have been shown to have coding potential, but their functionality is largely uncharacterized. Less than 2% of the human genome sequences encode for proteins.
ncRNAs do not undergo translation, but they are believed to serve as regulatory elements in the genome. Thus, they could hold the key to broadening our understanding of gene regulation in the context of human disease. Examples of small ncRNAs include ribosomal RNA (rRNA), transfer RNA (tRNA), small nuclear RNA (snRNA), piwi-interacting RNA (piRNA), micro-RNA (miRNA), and silencing RNA (siRNA).
This diversity provides huge potential but also brings interesting challenges for manufacturers, as it may be that different molecules are suitable for different therapeutics. For example, therapeutic modalities for mRNA include replacement therapy, vaccination, and cell therapy [16]Damase TR, Sukhovershin R, Boada C, Taraballi F, Pettigrew RI, Cooke JP. The Limitless Future of RNA Therapeutics. Front. Bioeng. Biotechnol. 2021; 9. .Therapies created from siRNA usually involve gene downregulation, miRNA target multiple genes within one pathway for broad but specific response, thus making them useful for cancer [17]Winkle M, El-Daly SM, Fabbri M et al. Noncoding RNA therapeutics – challenges and potential solutions. Nat. Rev. Drug Discov. 2021; 20, 629–651. .
Most mRNA vaccines in clinical trials today are the traditional non-replicating type. Non-replicating mRNA vaccines are transient by nature and typically express antigen for a few hours or days. For some applications this can be beneficial; however, for others, such as systemic protein therapies, extended expression of a protein would be beneficial [18]Yılmaz E, Aşı Teknolojisinde, Yeni Umutlar. mRNA Aşıları (New Hopes in Vaccine Technology: mRNA Vaccines). However, saRNA can deliver genes, such as a viral RNA polymerase, to enable mRNA to self-replicate. While this requires delivery of a more complex and longer RNA molecule, it could provide greatly enhanced biological activity, which allows for lower doses [19]. Mikrobiyol. Bul. 2021; 55(2), 265–284. Turkish. . CircRNA is attractive for its increased stability, allowing rapid production via in vitro transcription without nucleotide modification, thus providing cost savings. However, at this time, it is still difficult to tell if it will offer more than linear mRNA. CircRNAs are expected to be potential biomarkers for many diseases, with recent advances shown in melanoma [20]Blakney AK, McKay PF, Bouton CR, Hu K, Samnuan K, Shattock RJ. Innate Inhibiting Proteins Enhance Expression and Immunogenicity of Self-Amplifying RNA. Mol. Ther. 2021; 29(3), 1174–1185..
mRNA technology is changing the way therapies are developed
The potential of mRNA vaccines gained scientific attention in 1990 after the in vivo expression of a protein was observed upon injection of naked mRNA into the skeletal muscle of a mouse [21]Keyun T, Zhang H, Yaqi L, Qiuning S, Hongzhong J. Circular RNA as a Potential Biomarker for Melanoma: A Systematic Review. Front. Cell Devel. Biol. 2021; 9. . Since then, the industry has seen rapid development and expansion. Today, more than 140 clinical trials have looked at mRNA to address infectious disease, cancer, and a variety of other
application areas.
While there are questions over the advantages and disadvantages of the types of mRNA, the overall potential is clear. mRNA therapeutics are currently being developed in many areas. The advances made in mRNA vaccines for infectious diseases are renowned, but less is known about RNA therapies that are being developed by reimagining what is possible with existing technologies, such as in vivo gene editing techniques, RNA cell therapy as a safer alternative to CAR T and using mRNA to deliver the sequence of an antibody as an alternative to cells. RNA technologies are also being researched for use in allergen-specific immunotherapy [22]Rosa SS, Prazeres DMF, Azevedo AM, Marques MPC. mRNA vaccines manufacturing: challenges and bottlenecks. Vaccine. 2021; 39(16), 2190–2200. as well as in agriculture as a vehicle to replace pesticides [23]Scheiblhofer S, Thalhamer J, Weiss R. DNA and mRNA vaccination against allergies. Pediatr. Allergy Immunol. 2018; 29(7), 679–688..
mRNA systems in comparison to traditional viral vector systems, are much faster as they do not require animal cells. This also potentially makes them safer, although mRNA vaccines have yet to have the proven efficacy and safety of viral vector vaccines due to their novelty. Many manufacturers do not wish to invest in additional technology to manufacture a new modality, particularly one that currently has increased logistical costs. However, mRNA vaccines have the potential to work for small- to medium-batch sizes as well as large-batch scale, making them suitable for personalized medicine [24]Shaffer L. RNA-based pesticides aim to get around resistance problems. Proc. Natl. Acad. Sci. USA. 2020; 117(52), 32823–32826..
New modality for cancer treatment
mRNA vaccines have also gained traction as a therapeutic approach for treating cancer. mRNA can elicit immune responses to mutated oncogenes or regulatory cancer genes such as TP53, which are shared across many cancers, in a therapeutic pan-cancer approach. Other approaches for cancer include personalized therapy, where vaccines are developed for a person’s individual mutations. In this regard, a patient’s mutanome would be identified by next-generation sequencing, and a handful of custom mRNA vaccines would be developed targeting the individual’s neoantigens [25]Sheridan C. mRNA printers kick-start personalized medicines for all. Nat. Biotechnol. 2022; 40, 1160–1162..
The speed and potential cost gains of mRNA technology make it an interesting technology for personalized medicine. It is possible to take tumor tissue samples and develop mRNA vaccines that result in the expression of tumor antigens [26]Vormehr M, Schrörs B, Boegel S, Löwer M, Türeci Ö, Sahin U. Mutanome engineered RNA immunotherapy: towards patient-centered tumor vaccination. J. Immunol. Res. 2015; 2015, 595363.. Many companies are working on integrated system mRNA processing solutions for this, such as CureVac with an mRNA printer and companies pursuing plug and play approaches like Nutcracker Therapeutics [24]Shaffer L. RNA-based pesticides aim to get around resistance problems. Proc. Natl. Acad. Sci. USA. 2020; 117(52), 32823–32826.. However, there is also room for improvement in smaller scale cGMP manufacturing, as much of the current equipment is repurposed from the biotech industry and is designed for much larger scales than needed for mRNA.
Therapeutic cancer vaccines are advancing quickly in development, with over 70 clinical trials completed and more results expected in the next two to three years [21]Keyun T, Zhang H, Yaqi L, Qiuning S, Hongzhong J. Circular RNA as a Potential Biomarker for Melanoma: A Systematic Review. Front. Cell Devel. Biol. 2021; 9. Keyun T, Zhang H, Yaqi L, Qiuning S, Hongzhong J. Circular RNA as a Potential Biomarker for Melanoma: A Systematic Review. Front. Cell Devel. Biol. 2021; 9. . Techniques under evaluation include the direct stimulation of antigen-presenting cells via ex vivo electroporation of mRNA. Other approaches include direct intratumor injection, whole body approaches, and targeted organ approaches. Currently over 50% of clinical trials using mRNA focus on the treatment of melanomas and prostate and brain cancer [21]Keyun T, Zhang H, Yaqi L, Qiuning S, Hongzhong J. Circular RNA as a Potential Biomarker for Melanoma: A Systematic Review. Front. Cell Devel. Biol. 2021; 9. Keyun T, Zhang H, Yaqi L, Qiuning S, Hongzhong J. Circular RNA as a Potential Biomarker for Melanoma: A Systematic Review. Front. Cell Devel. Biol. 2021; 9. . Thus, while numerous applications of mRNA vaccines are in various stages of development, targeting specific organs, tissues, and cells with lipid nanoparticles (LNPs) is still under research.
Considerations for rapid pandemic response
In an article for The New England Journal of Medicine [27]Parums DV. Editorial: mRNA Vaccines and Immunotherapy in Oncology: A New Era for Personalized Medicine. Med. Sci. Monit. 2021; 27, e933088., several doctors and scientists who work at CEPI wrote that there are five categories to focus on to enable a rapid vaccine strategy:
- Leveraging insights about new pathogens and technologies;
- Supporting innovation in the vaccine development process;
- Advancing analytics to inform processes;
- Promoting collaboration among stakeholders;
- Continuously reviewing evidence to support approval
Implementing current best practices while leveraging these goals could enable developing pandemic vaccines in 100 days. To allow rapid testing of candidates, the National Institute of Allergy, and Infectious Diseases (NIAID) proposed to develop and characterize prototype vaccines [28]Saville M, Cramer JP, Downham M et al. Delivering pandemic vaccines in 100 days–what will it take? N. Engl. J. Med. 2022; 387(2), e3. [29]Graham BS, Sullivan NJ. Emerging viral diseases from a vaccinology perspective: preparing for the next pandemic.Nat. Immunol. 2018; 19(1), 20–28.. The goals for the NIAID Preparedness Plan are similar in scope, proposing to [30]Bok K, Sitar S, Graham BS, Mascola JR. Accelerated COVID-19 vaccine development: milestones, lessons, and prospects.Immunity. 2021; 54(8), 1636–1651.:
- Characterize pathogens of concern;
- Shorten timelines between pathogen emergence and countermeasures;
- Bridge or eliminate gaps in research, infrastructure, and technology
Key findings from previous pandemics and epidemics indicate a lack of existing diagnostics, therapeutics, and vaccines, low manufacturing capacity; process efficiencies, and a lack of coordination and preparedness [31]National Institute of Allergy and Infectious Diseases. Pandemic Preparedness. National Institute of Allergy and Infectious Diseases. (Accessed July 25, 2022).. The 100-day mission suggests that embedding best practices and preparation into usual process measures is needed, for example, by making simplified and transferable manufacturing practices the norm and enabling scaled-up processes when needed.
With several choices available, it may be that manufacturers of therapeutics will need to adopt a flexible approach to manufacturing, as one modality could suit a specific therapeutic better than another. At this time, manufacturers may find themselves with a choice to stay with a specific modality (e.g., mRNA vaccines, viral vector vaccines) or focus on a specific research area and adapt manufacturing to what is needed. This requires a degree of resilience in manufacturing strategies.
The timeline for manufacturing and release of a clinical-grade vaccine will always be platform dependent, but mRNA vaccines offer the potential to be completed in as little as five weeks [27]Parums DV. Editorial: mRNA Vaccines and Immunotherapy in Oncology: A New Era for Personalized Medicine. Med. Sci. Monit. 2021; 27, e933088., in comparison to viral vector systems which can take around 6–36 months [21]Keyun T, Zhang H, Yaqi L, Qiuning S, Hongzhong J. Circular RNA as a Potential Biomarker for Melanoma: A Systematic Review. Front. Cell Devel. Biol. 2021; 9. . The increased speed from discovery to delivery for mRNA vaccines, will enable manufacturers to bring vaccines to the market quicker. This allows the technology to be suitable for meeting the 100-day strategy timeline. However, to be able to achieve this, manufacturers need to enhance their platforms now for rapid scale-up potential.
Overcoming manufacturing challenges
While there are many challenges to manufacturing a therapeutic, a key challenge is adapting processes. Often, solutions are home-built or optimized for other molecules and distributed processes can lead to bottlenecks. Many manufacturers don’t have as much knowledge of these new arenas of manufacturing development and so they have not yet been standardized. This leads to challenges with operations, personnel, process, quality control, contamination, and others.
Process is central to biomanufacturing. A biomanufacturing enterprise includes processing parameters, facility, resources, and infrastructure; these elements are integrated and influence each other. When assessing vaccine manufacturing, many of the technical operations will be translatable, regardless of modality, especially when following GMP manufacturing guidance. While some processes will be different for each modality, there will also be many similarities in operations, such as the equipment used for purification and in vitro transcription (IVT). Holistic solutions can reduce project risks, stabilize costs, maximize capacity, and help speed up time to market.
One challenge for mRNA manufacturing is that it is much smaller scale than traditional cell-based modality manufacturing, requiring manufacturers to think differently about their space. However, this is one of the reasons why mRNA is so attractive, as it can provide considerable space and cost savings. Changing parameters in cell-based manufacturing usually results in time delays, whereas in mRNA manufacturing this is much quicker and doesn’t have as many contamination issues to address. This makes mRNA vaccines suitable for the 100-day strategy.
Process challenges for mRNA include DNA linearization and purity at various stages of the process. Purification can be more challenging for mRNA molecules because, due to their size and varying impurity profiles, they do not interact well with traditional chromatography resins. Flexibility in purification technologies or allowing process development scientists to mix and match media based on the specific characteristics the molecule, may help alleviate this problem. Other challenges can include obtaining GMP-grade reagents and ensuring continuous supply of raw materials.
Flexibility to scale is key to success
With more modality and scale diversity than ever before, it’s important to build in flexible and resilient solutions that allow researchers the ability to scale, and scale rapidly, if needed. One of the most common bottlenecks in the current manufacturing of mRNA is scaling, with mass population vaccines needing larger-scale production technology. Manufacturers can build flexibility into their processes by ensuring that equipment is scalable and supports the transfer from process development to GMP manufacturing. Flexible, configurable manufacturing solutions, such as the Cytiva FlexFactory™ platform and KUBio™ modular facilities, offer full start-to-finish, tailored solutions, developed and delivered for monoclonal antibody (mAb) applications as well as for plasmids, mRNA, and viral vectors.
One way to look at the scale of manufacturing is to estimate market size, the uptake of a potential therapy, and the dosing strategy, and then back calculate from that. Investing in flexibility will make it possible to grow, allowing a small fast start with something that is easy to scale up or scale out. Considering manufacturability and scale-up from the beginning can avoid problems later in the process. A protocol or method that quickly gives a pure product for early clinical material might be great, but if it can’t scale up or be used in manufacturing, this will become an issue.
Manufacturability includes assessing material suitability and thinking about material sourcing early. Continuous planning, including maintaining a close relationship and regular communication with suppliers, is critical for rapid expansion of manufacturing facilities. It is also essential to know the quality attributes of the product, so early thinking on QC testing can save time later in the process.
Targeted delivery of mRNA therapeutics is an issue that needs to be addressed, as mRNA is unstable and needs protection from RNAse degradation. Delivery methods include lipid-based nanoparticles (LNPs), polymer nanomaterials, silica nanoparticles, carbon and gold nanomaterials and N-Acetylgalactosamine (GalNAc). Examples of proven efficacy of the LNP platform include patisirin and Alnylam and the COVID-19 vaccine from BioNTech and Moderna [16]Damase TR, Sukhovershin R, Boada C, Taraballi F, Pettigrew RI, Cooke JP. The Limitless Future of RNA Therapeutics. Front. Bioeng. Biotechnol. 2021; 9. .
LNPs are typically formed in a rapid mixing process using microfluidic devices [32]UK Government. 100 Days Mission to respond to future pandemic threats - A report to the G7 by the pandemic preparedness partnership. UK government 2021. (Accessed July 25, 2022).UK Government. 100 Days Mission to respond to future pandemic threats - A report to the G7 by the pandemic preparedness partnership. UK government 2021. (Accessed July 25, 2022)., which is more of an art than an established method. Greater understanding of the influences of the LNP ingredients and their effect on LNP stability, delivery, efficiency, immune response, and ultimately patient outcomes would benefit the industry [32]UK Government. 100 Days Mission to respond to future pandemic threats - A report to the G7 by the pandemic preparedness partnership. UK government 2021. (Accessed July 25, 2022).UK Government. 100 Days Mission to respond to future pandemic threats - A report to the G7 by the pandemic preparedness partnership. UK government 2021. (Accessed July 25, 2022).. Optimization of LNPs and other delivery technologies is critical to determining the ultimate success or failure of a therapeutic.
Filling and finishing of vaccines will also need to be considered for rapid response. Distribution can be an issue, as current mRNA vaccines require frozen storage. Alternative methods, such as lyophilization, are under study [33]Kim J, Eygeris Y, Gupta M, Sahay G. Self-assembled mRNA vaccines. Adv. Drug Deliv. Rev. 2021; 170, 83–112. , with Gennova recently achieving approval for its COVID-19 vaccine in powder form [34]Xu S, Yang K, Li R, Zhang L. mRNA vaccine era–mechanisms, drug platform and clinical prospection. Int. J. Mol. Sci. 2020; 21(18), 6582..
The ethos of flexible and scalable solutions is something that Cytiva promotes across many product ranges, allowing support of manufacturing in various modalities, including non-viral delivery for genomic medicines, and at different scales (Figure 2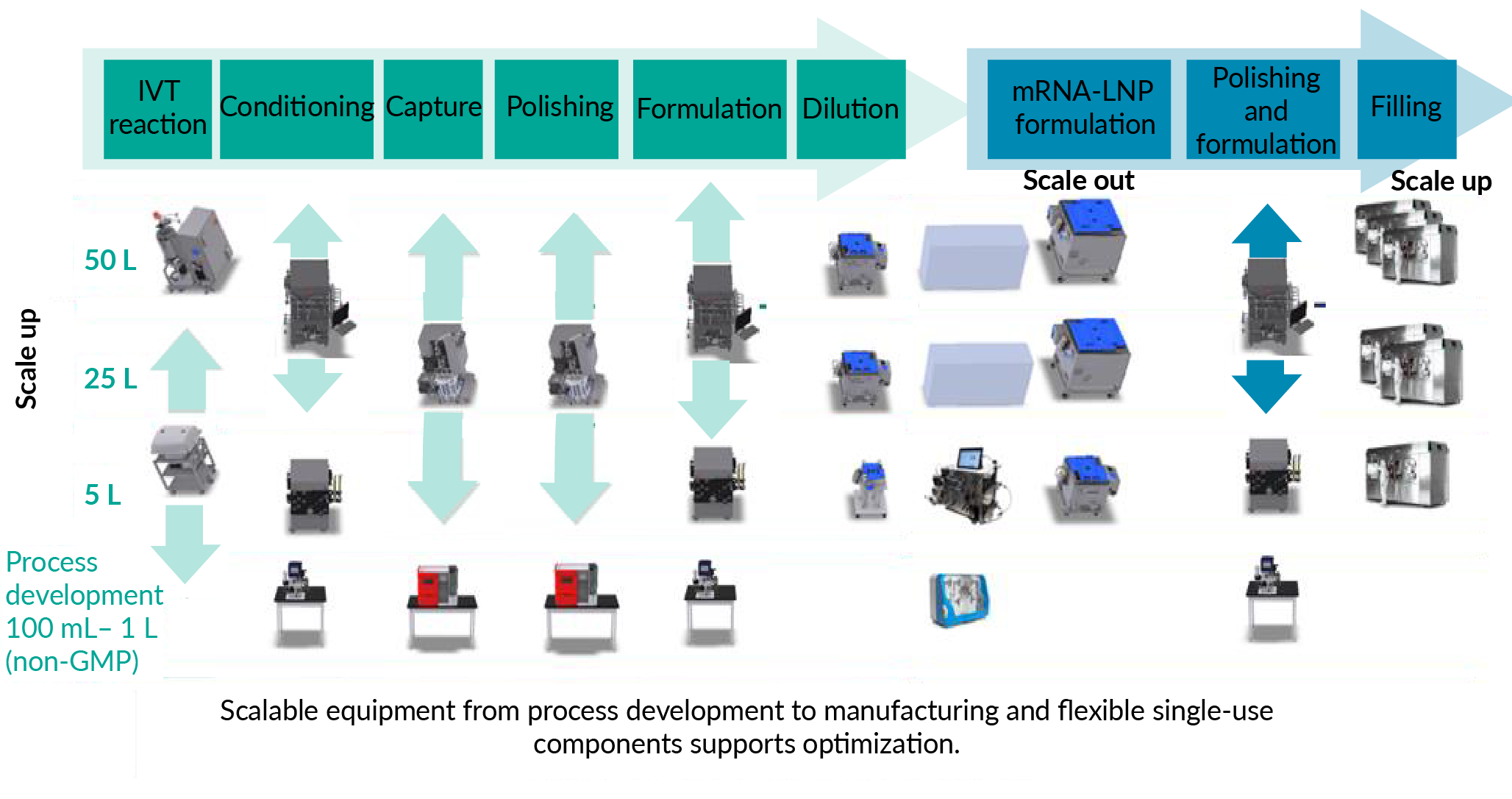 Scalable equipment, from process development to manufacturing and flexible single-use components, support optimization.). Manufacturers can be prepared for future pandemics by building flexibility into their processes, helping to ensure that equipment is scalable and supports the transfer from process development to GMP manufacturing at a rapid pace.
Scalable equipment, from process development to manufacturing and flexible single-use components, support optimization.). Manufacturers can be prepared for future pandemics by building flexibility into their processes, helping to ensure that equipment is scalable and supports the transfer from process development to GMP manufacturing at a rapid pace.
RNA technology & the potential of the 100-day vaccine
Currently, mRNA vaccines are one of the most suitable manufacturing modalities to meet the 100-day vaccine strategy, due to the increased speed they offer during the manufacturing stage. RNA therapeutics is a rapidly growing field, with many applications in development and therapeutics in clinical trials. The technology offers great potential in the areas of infectious disease and personalized medicine due to its advantages in flexibility, cost, and speed of development. There are still challenges to overcome to fully realize the possibilities, but some of these will be addressed with increased focus and funding.
References
1. CEPI. 100 Days. CEPI 2022. (Accessed July 2022). Crossref
2. Cornish L. Who’s funding the COVID-19 response and what are the priorities? Devex (Jul 21 2021) (Accessed September 2022). Crossref
3. NIH. The Human Genome Project. NIH. (Accessed September 2022). Crossref
4. Sridharan K, Gogtay NJ. Therapeutic nucleic acids: current clinical status. Br. J. Clin. Pharmacol. 2016; 82(3), 659–672. Crossref
5. Karadima S, Vaidya M. Foreign investments amplify the nucleic acid therapeutics field. Pharmaceutical Technology (Aug 25 2022). (Accessed September 2022). Crossref
6. Merck. Merck and Orna Therapeutics Collaborate to Advance Orna’s Next Generation of RNA Technology. Merck (Aug 16 2022). (Accessed September 2022. Crossref
7. Lilly Investors. Lilly and MiNA Therapeutics Announce saRNA Research Collaboration. Lilly Investors (May 11 2021)(Accessed September 2022). Crossref
8. Lilly Investors. Lilly Announces the Institute for Genetic Medicine and $700 Million investment in Boston Seaport Site. Lilly Investors (Feb 22 2022). (Accessed September 2022). Crossref
9. Laws L. Millions in funding and new board members for Belgian mRNA company. LABIOTEC.eu (Aug 24 2022). (Accessed September 2022). Crossref
10. yahoo!finance. Arcturus Announces $63.2 Million Award from the U.S. Government to Support Development of Self-amplifying mRNA Vaccine for Rapid Pandemic Influenza Response. yahoo!finance (Aug 31 2022). (Accessed September 2022). Crossref
11. Bell J. Next-generation RNA technologies: making longer-lasting drugs with a broader reach. BIOPHARMADIVE * Accessed September 2022. (Aug 23 2022). (Accessed September 2022). Crossref
12. Kutter C, Svoboda P. miRNA, siRNA, piRNA: Knowns of the unknown. RNA Biol. 2008; 5(4), 181–188. Crossref
13. Statello L, Guo CJ, Chen LL et al. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021; 22, 96–118. Crossref
14. Karikó K. In vitro-Transcribed mRNA Therapeutics: Out of the Shadows and Into the Spotlight. Mol. Ther. 2019; 27(4), 691–692. Crossref
15. Cécile J, Bilal BM, Romano R. Emerging Classes of Small Non-Coding RNAs With Potential Implications in Diabetes and Associated Metabolic Disorders. Front. Endocrinol. 2021; 12. Crossref
16. Damase TR, Sukhovershin R, Boada C, Taraballi F, Pettigrew RI, Cooke JP. The Limitless Future of RNA Therapeutics. Front. Bioeng. Biotechnol. 2021; 9. Crossref
17. Winkle M, El-Daly SM, Fabbri M et al. Noncoding RNA therapeutics – challenges and potential solutions. Nat. Rev. Drug Discov. 2021; 20, 629–651. Crossref
18. Yılmaz E, Aşı Teknolojisinde, Yeni Umutlar. mRNA Aşıları (New Hopes in Vaccine Technology: mRNA Vaccines) Crossref
19. . Mikrobiyol. Bul. 2021; 55(2), 265–284. Turkish. Crossref
20. Blakney AK, McKay PF, Bouton CR, Hu K, Samnuan K, Shattock RJ. Innate Inhibiting Proteins Enhance Expression and Immunogenicity of Self-Amplifying RNA. Mol. Ther. 2021; 29(3), 1174–1185. Crossref
21. Keyun T, Zhang H, Yaqi L, Qiuning S, Hongzhong J. Circular RNA as a Potential Biomarker for Melanoma: A Systematic Review. Front. Cell Devel. Biol. 2021; 9. Crossref
22. Rosa SS, Prazeres DMF, Azevedo AM, Marques MPC. mRNA vaccines manufacturing: challenges and bottlenecks. Vaccine. 2021; 39(16), 2190–2200. Crossref
23. Scheiblhofer S, Thalhamer J, Weiss R. DNA and mRNA vaccination against allergies. Pediatr. Allergy Immunol. 2018; 29(7), 679–688. Crossref
24. Shaffer L. RNA-based pesticides aim to get around resistance problems. Proc. Natl. Acad. Sci. USA. 2020; 117(52), 32823–32826. Crossref
25. Sheridan C. mRNA printers kick-start personalized medicines for all. Nat. Biotechnol. 2022; 40, 1160–1162. Crossref
26. Vormehr M, Schrörs B, Boegel S, Löwer M, Türeci Ö, Sahin U. Mutanome engineered RNA immunotherapy: towards patient-centered tumor vaccination. J. Immunol. Res. 2015; 2015, 595363. Crossref
27. Parums DV. Editorial: mRNA Vaccines and Immunotherapy in Oncology: A New Era for Personalized Medicine. Med. Sci. Monit. 2021; 27, e933088. Crossref
28. Saville M, Cramer JP, Downham M et al. Delivering pandemic vaccines in 100 days–what will it take? N. Engl. J. Med. 2022; 387(2), e3. Crossref
29. Graham BS, Sullivan NJ. Emerging viral diseases from a vaccinology perspective: preparing for the next pandemic.Nat. Immunol. 2018; 19(1), 20–28. Crossref
30. Bok K, Sitar S, Graham BS, Mascola JR. Accelerated COVID-19 vaccine development: milestones, lessons, and prospects.Immunity. 2021; 54(8), 1636–1651. Crossref
31. National Institute of Allergy and Infectious Diseases. Pandemic Preparedness. National Institute of Allergy and Infectious Diseases. (Accessed July 25, 2022). Crossref
32. UK Government. 100 Days Mission to respond to future pandemic threats - A report to the G7 by the pandemic preparedness partnership. UK government 2021. (Accessed July 25, 2022). Crossref
33. Kim J, Eygeris Y, Gupta M, Sahay G. Self-assembled mRNA vaccines. Adv. Drug Deliv. Rev. 2021; 170, 83–112. Crossref
34. Xu S, Yang K, Li R, Zhang L. mRNA vaccine era–mechanisms, drug platform and clinical prospection. Int. J. Mol. Sci. 2020; 21(18), 6582. Crossref
35. Gennova. mRNA Based Vaccines. Gennova. (Accessed September 2022). Crossref
Affiliation
Tracy Humphries
Global mRNA Segment Marketing Leader,
Cytiva
Authorship & Conflict of Interest
Contributions: The named authors takes responsibility for the integrity of the work as a whole, and has given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: The author has no conflicts of interest.
Funding declaration: The author received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Vaccine Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2022 Cytiva. Published by Vaccine Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Aug 11 2022; Revised manuscript received: Oct 19 2022; Publication date: Nov 10 2022.

