Development of recombinant vesicular stomatitis virus vaccine platform for rapid response to Ebola and COVID-19 outbreaks
Vaccine Insights 2023; 2(5), 181–190
DOI: 10.18609/vac/2023.029
Epidemic and pandemic outbreaks can increase mortality, cause upheaval to healthcare systems, and disrupt global economy and security. Given these threats, it is imperative that there are rapid responses to outbreaks to limit human, social, and economic costs of pandemics. The Ebola virus disease (EVD) epidemic and COVID-19 pandemic posed serious threats to global health, affecting millions to billions of people and disrupting public health services worldwide. Although the viruses associated with EVD and COVID-19 have demonstrated strong infectivity, the high fatality rate of EVD has restricted its spread and prevented it from reaching pandemic level. The responses to the Ebola virus and SARS-CoV-2 outbreaks from manufacturers such as Merck & Co., Inc., Rahway, NJ, USA (MSD) have pushed the boundaries for vaccine development in several areas, including accelerated, parallel clinical and commercial development timelines, implementation of single-use tech-nologies in manufacturing, and engagement with partners and regulatory agencies globally. This review describes how MSD 1) applied the recombinant vesicular stomatitis virus (rVSV) vaccine platform to quickly develop a vaccine for Ebola virus and 2) applied both the rVSV platform and prior knowledge gained from development of the Ebola virus vaccine to rapidly respond to the SARS-CoV-2 pandemic.
Platform process development & manufacturing
Among the various vaccine platforms, live virus vaccines (LVV) are considered the most effective at eliciting life-long cellular and humoral immune responses [1]Al-Jighefee HT, Najjar H, Ahmed MN, Qush A, Awwad S, Kamareddine L. COVID-19 Vaccine Platforms: Challenges and Safety Contemplations. Vaccines 2021; 9(10), 1196. . However, developing a manufacturing platform for LVV is challenging since different virus families may require different cell substrates, production processes, and purification requirements. LVV can be either attenuated strains or recombinant strains; live recombinant vaccines are replicating viruses that are genetically engineered to carry heterologous antigens. One advantage of live recombinant viruses is that the presentation of heterologous proteins in combination with mimicry of natural infection from live viral vector can generate strong humoral and cellular immune responses without an adjuvant [2]Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments [published correction appears in Nat. Rev. Immunol. 2021 Jan 5]. Nat. Rev. Immunol. 2021; 21(2), 83–100. . In the past decade, rVSV has been established as a live recombinant vaccine platform for multiple viral diseases [3]Scher G, Schnell MJ. Rhabdoviruses as vectors for vaccines and therapeutics. Curr. Opin. Virol. 2020; 44, 169–182. .
VSV is a member of the Rhabdoviridae family of negative-stranded RNA viruses and causes non-lethal disease in cattle, horses, and pigs; human VSV infections are rare [4]Rose JK, Schubert M. Rhabdovirus genomes and their products. In: The Rhabdoviruses (Editor: Wagner RR). 1987; 129–166, Plenum Publishing Corporation.. rVSV was first developed as a replicating vaccine platform by John Rose and Michael Whitt [5]Lawson ND, Stillman EA, Whitt MA, Rose JK. (1995). Recombinant vesicular stomatitis viruses from DNA. Proc. Nat. Acad. Sci. USA 1995; 92(10), 4477–4481. [6]Whitt MA. Generation of VSV pseudotypes using recombinant ΔG-VSV for studies on virus entry, identification of entry inhibitors, and immune responses to vaccines. J. Virol. Methods 2010; 169(2), 365–374. . Many aspects of the rVSV are advantageous for vaccine development:
- VSV can be propagated to high titers in many cell lines;
- VSV elicits strong cellular and humoral immunity in vivo;
- The VSV-G protein, the major virulence factor of VSV, can be eliminated, thus attenuating the virus and reducing its reactogenicity [7];
- There is a low prevalence of immunity to VSV in most of the general population, making it advantageous to use rVSV as a vaccination platform;
- VSV replicates within the cytoplasm of infected cells and does not integrate into the host genome, reducing the risk of oncogenesis and mutagenesis [8].
Vero cells have been the workhorse for vaccine production over the past 40 years. The cell line was established from cells isolated from a kidney of a normal African green monkey [9]Yasumura Y, Kawakita Y. Studies on SV40 in tissue culture-preliminary step for cancer research in vitro. Nihon Rinsho. 1963; 21(21), 1201–1215.. Vero cells are one of the most common continuous cell lines used for vaccine production; they have been extensively characterized and have gained global acceptance by regulatory authorities [10]Barrett PN, Mundt W, Kistner O, Howard MK. Vero cell platform in vaccine production: moving towards cell culture-based viral vaccines. Exp. Rev. Vaccines 2009; 8(5), 607–618. . Vero cells do not produce type I interferon in response to viral infections [11]Desmyter J, Melnick JL, Rawls WE. Defectiveness of interferon production and of rubella virus interference in a line of African green monkey kidney cells (Vero). J. Virol. 1968; 2(10), 955–961. , which may explain the susceptibility of these cells to many viruses. This broad susceptibility of Vero cells to many viruses makes them an ideal cell substrate for the development and production of viral vaccines.
Ebola vaccine
On August 8, 2014, the WHO declared the EVD outbreak in West Africa a Public Health Emergency of International Concern [12]World Health Organization (WHO), WHO Statement on the Meeting of the International Health Regulations Emergency Committee Regarding the 2014 Ebola Outbreak in West Africa, 2014.. Understanding the urgency of developing an effective vaccine for Ebola virus, NewLink Genetics Corp., in partnership with the US FDA, reached out to MSD to develop an rVSV Ebola vaccine candidate. With extensive internal knowledge of developing LVV, working experience with Vero cells, and scaling up viral vaccine production, MSD partnered with NewLink Genetics Corporation to develop the Ebola vaccine manufacturing process. Leveraging data from the Public Health Agency of Canada, NewLink Genetics and contract manufacturer IDT Biologika, existing literature for rVSV, and extensive internal knowledge of scaling up vaccine production with Vero cells, MSD initiated process development of a robust and scalable manufacturing process.
MSD was challenged with scaling up the existing Ebola rVSV process from 90 to 400 roller bottles to meet Pre-Licensed Patient Access (PLPA) needs. Understanding that the process had to be scaled up quickly, Vero cell expansion experience from the RotaTeq® vaccine was leveraged to develop the Ebola vaccine process. There were two main areas of focus:
- Infection and harvest parameters;
- Scalable downstream unit operations.
To accomplish this, the development team performed repeated cell expansions to generate material to initiate harvest/infection and downstream experiments. Specifically, increased filter surface area, a new tangential flow filter scheme, decreased lumen diameter to maintain shear, and reduced circulation rate was implemented.
Providing significant starting material to these teams was imperative to allow the creation of multiple side-arm experiments to test various process changes simultaneously in parallel experimental arms. This methodology also provided opportunities to complete non-GMP full-scale runs on the upstream process in the pilot plant facility, allowing electronic notebook documentation to later be adapted to production batch records. Experiments were led by a pilot plant operations team, leveraging experience from team members who had previously worked in biologic and vaccine process development areas. The multitude of small-scale purification runs provided hands-on experience to the team that would later be tasked with scale-up for GMP production. Co-locating process development and GMP clinical manufacturing in the same organization with the same scientists eliminated the need for tech transfer.
To accelerate the manufacture of drug substance for Ebola vaccine, the development timeline was drastically compressed (Figure 1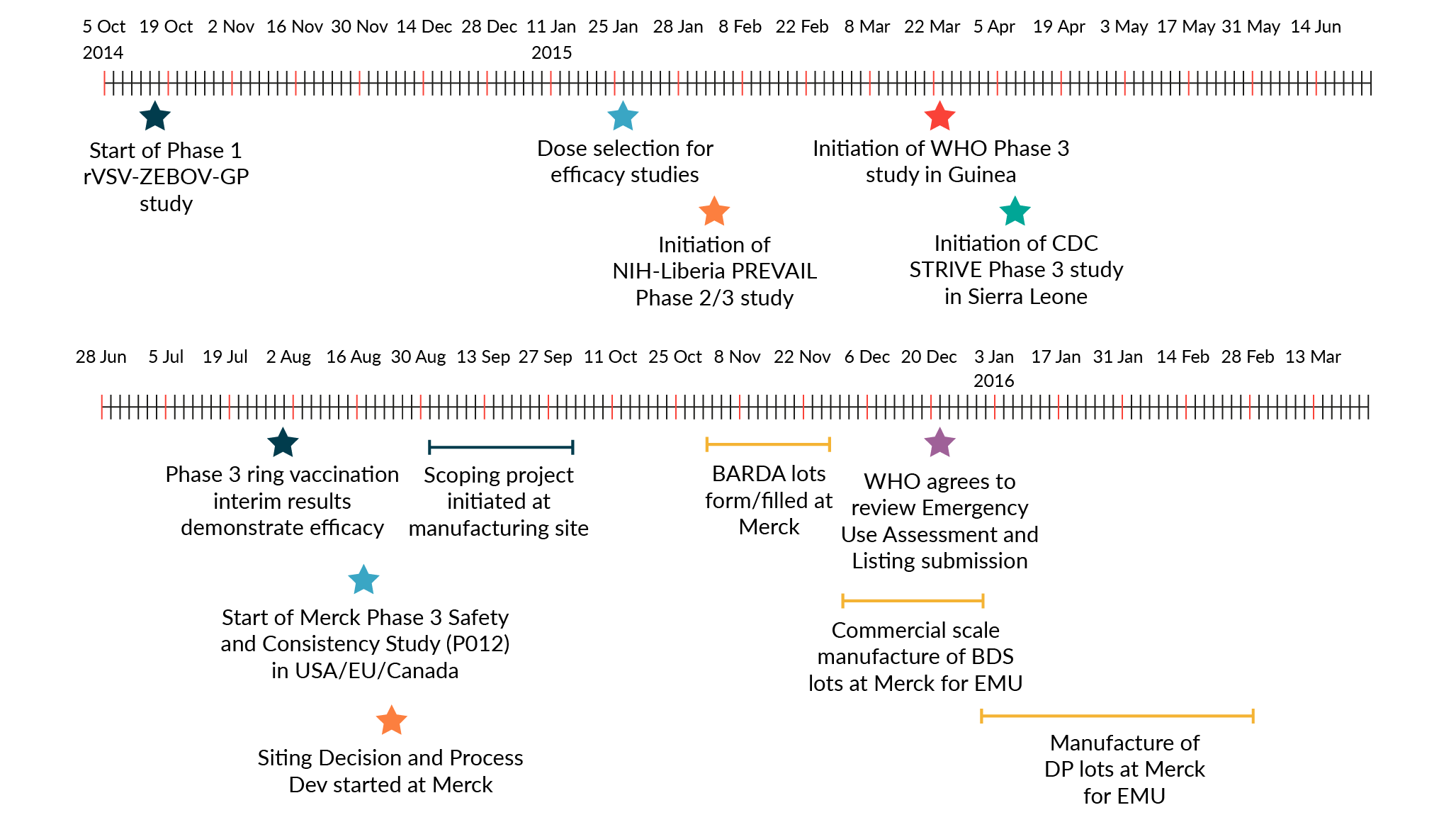 ERVEBO® development timeline. BARDA: Biomedical Advanced Research and Development Authority; BDS: Bulk drug substance; CDC: Centers for Disease Control and Prevention; DP: Drug product; EMU: European Medicines Agency; NIH: National Institute of Health; WHO: World Health Organization.). The time from initiation of process development activities at MSD to completion of manufacture for the first batch of GMP PLPA drug substance was 7 months. MSD was able to shorten the development timeline for rapid transfer to manufacturing by executing development and manufacturing scale-up activities in parallel and by implementing single-use technologies. The 400-roller bottle manufacturing process, while not state of the art, was completely disposable end-to-end. Single-use systems provided agility and scalability in a manufacturing facility. Different single-use systems at different scales were installed, commissioned, or removed quickly to meet production requirements. Furthermore, the use of single-use systems reduced manufacturing timelines via the elimination of cleaning validation, clean-in-place, and sterilization-in-place.
ERVEBO® development timeline. BARDA: Biomedical Advanced Research and Development Authority; BDS: Bulk drug substance; CDC: Centers for Disease Control and Prevention; DP: Drug product; EMU: European Medicines Agency; NIH: National Institute of Health; WHO: World Health Organization.). The time from initiation of process development activities at MSD to completion of manufacture for the first batch of GMP PLPA drug substance was 7 months. MSD was able to shorten the development timeline for rapid transfer to manufacturing by executing development and manufacturing scale-up activities in parallel and by implementing single-use technologies. The 400-roller bottle manufacturing process, while not state of the art, was completely disposable end-to-end. Single-use systems provided agility and scalability in a manufacturing facility. Different single-use systems at different scales were installed, commissioned, or removed quickly to meet production requirements. Furthermore, the use of single-use systems reduced manufacturing timelines via the elimination of cleaning validation, clean-in-place, and sterilization-in-place.
Prior to the partnership with MSD, IDT Biologika had initiated a Phase 1 clinical study utilizing material from their existing roller bottle process. In order to use the data from this ongoing study for lot consistency, MSD could not deviate from the roller bottle process to deliver PLPA material. Only changes that supported an increased manufacturing scale were evaluated. For upstream, the process was scaled from 90 to 400 roller bottles to produce the necessary drug substance volume sought for PLPA use. The optimal multiplicity of infection and time of harvest were also determined for the scaled-up process. For downstream, loading studies were performed on the clarification filter to minimize surface area and properly size the filter area needed at larger scale. Range-finding experiments were conducted on the enzyme treatment step in an attempt to reduce the amount of Benzonase® endonuclease used in the process, thereby reducing cost. Temperature studies were conducted to evaluate if simpler, room-temperature manufacturing operations could be utilized. A constant volume ultrafiltration/diafiltration process was implemented to keep process volumes low and reduce manufacturing times. Multiple ultrafiltration filters were evaluated to replace the existing filter, which was not available at the increased scale.
Parallel process development, scale-up, and process transfer to the clinical manufacturing facility also enabled rapid Ebola vaccine development. As each process step was defined, GMP batch records were created by the same engineers, leveraging their experimental knowledge and experience. This brought speed and accuracy to the authoring process. Large-scale roller bottle processes, particularly at the scale demonstrated here, were manual in nature and required intensive hands-on training for execution. The pilot plant operations staff quickly recruited and upskilled new contract staff members to support clinical GMP manufacturing operations. After completing training, these staff members were assigned to help complete experimental work, later transferring these important skills to GMP production.
MSD initiated a Phase 1 clinical trial in fourth quarter 2014, and a Phase 2/3 and consistency lot studies were initiated in February and April 2016. Clinical efficacy data was obtained in June 2016, and ERVEBO® was licensed by the FDA in 2019 [13]Wolf J, Jannat R, Dubey S et al. Development of Pandemic Vaccines: ERVEBO Case Study. Vaccines (Basel) 2021; 9(3), 190. . Prior to licensure, the VSV Ebola vaccine was deployed in Guinea in 2015 during the West African Ebola epidemic and the 2018-2020 Democratic Republic of Congo outbreak using the PLPA process, demonstrating 100 and 97.5% efficacy respectively after a single dose [14]Henao-Restrepo AM, Longini IM, Egger M et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 2015; 386(9996), 857–866. [15]Henao-Restrepo AM, Camacho A, Longini IM et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!) [published correction appears in Lancet. 2017 Feb 4;389(10068):504] Lancet 2017; 389(10068), 505–518. . The success of ERVEBO demonstrated the effectiveness of the rVSV vaccine platform in pandemic settings.
COVID-19 vaccine
In response to the COVID-19 outbreak, MSD and the International AIDS Vaccine Initiative (IAVI) applied the rVSV vaccine platform to develop V590, a vaccine candidate for SARS-CoV-2 (Figure 2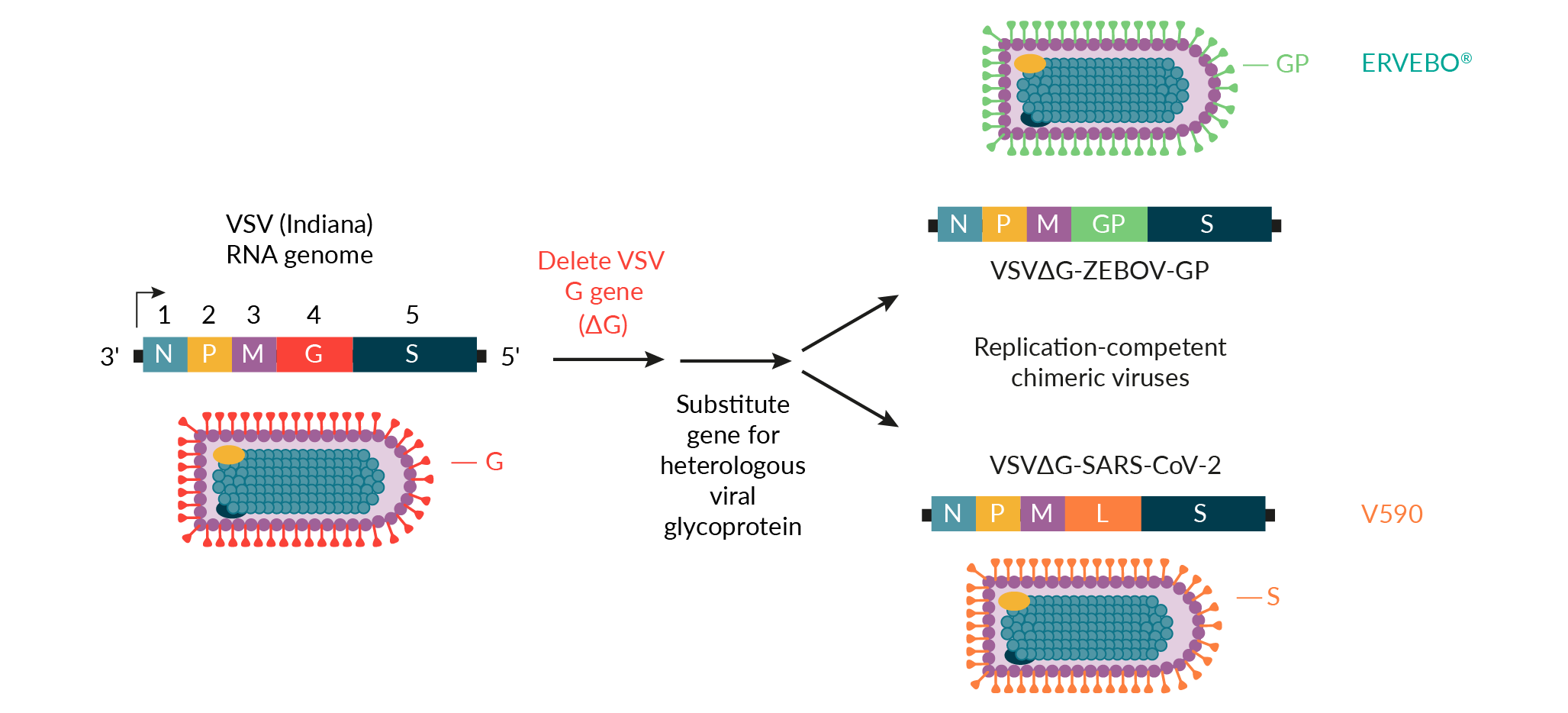 rVSV vaccine platform for the development of ERVEBO® and V590.VSV: Vesicular stomatitis virus.) [16]Saville M, Cramer JP, Downham M et al. Delivering pandemic vaccines in 100 days – what will it take? N. Engl. J. Med. 2022; 387, e3. . Early integration and real-time data sharing between discovery and process development teams at MSD enabled clone selection for optimal antigenicity and manufacturability. The use of the ERVEBO vaccine production platform also reduced the time required for V590 process development prior to the production of Phase 1 clinical supplies. For example, the ERVEBO upstream roller bottle process, with minor modifications, was leveraged for the production of V590 Phase 1 clinical supplies. The number of roller bottles was increased from 400 to 600 to ensure a sufficient supply of drug substance for Phase 1 clinical trials, and the infection time was reduced by roughly 12−24 hours compared to the ERVEBO process. While several downstream purification process steps were adopted directly from the ERVEBO process, differences between the VSV∆G-ZEBOV-GP and VSV∆G-SARS-CoV-2 viruses required the removal of the ERVEBO protease incubation step with TrypLE™ from the V590 process. This ultimately led to the inclusion of an aseptic, flow-through chromatography step using gamma-irradiated, sterilized Capto™Core 700 resin (Cytiva) to increase clearance of residual host cell proteins. A change to the final drug substance buffer was also incorporated to allow for improved V590 drug product shelf-life.
rVSV vaccine platform for the development of ERVEBO® and V590.VSV: Vesicular stomatitis virus.) [16]Saville M, Cramer JP, Downham M et al. Delivering pandemic vaccines in 100 days – what will it take? N. Engl. J. Med. 2022; 387, e3. . Early integration and real-time data sharing between discovery and process development teams at MSD enabled clone selection for optimal antigenicity and manufacturability. The use of the ERVEBO vaccine production platform also reduced the time required for V590 process development prior to the production of Phase 1 clinical supplies. For example, the ERVEBO upstream roller bottle process, with minor modifications, was leveraged for the production of V590 Phase 1 clinical supplies. The number of roller bottles was increased from 400 to 600 to ensure a sufficient supply of drug substance for Phase 1 clinical trials, and the infection time was reduced by roughly 12−24 hours compared to the ERVEBO process. While several downstream purification process steps were adopted directly from the ERVEBO process, differences between the VSV∆G-ZEBOV-GP and VSV∆G-SARS-CoV-2 viruses required the removal of the ERVEBO protease incubation step with TrypLE™ from the V590 process. This ultimately led to the inclusion of an aseptic, flow-through chromatography step using gamma-irradiated, sterilized Capto™Core 700 resin (Cytiva) to increase clearance of residual host cell proteins. A change to the final drug substance buffer was also incorporated to allow for improved V590 drug product shelf-life.
Though it was recognized that a roller bottle process with aseptic downstream processing would not be used for commercial-scale production due to the large number of anticipated doses for a COVID-19 vaccine, this fit-for-purpose approach allowed for rapid production of Phase 1 clinical supplies. Phase 1 clinical supplies were generated approximately 2 months after V590 clone selection (Figure 3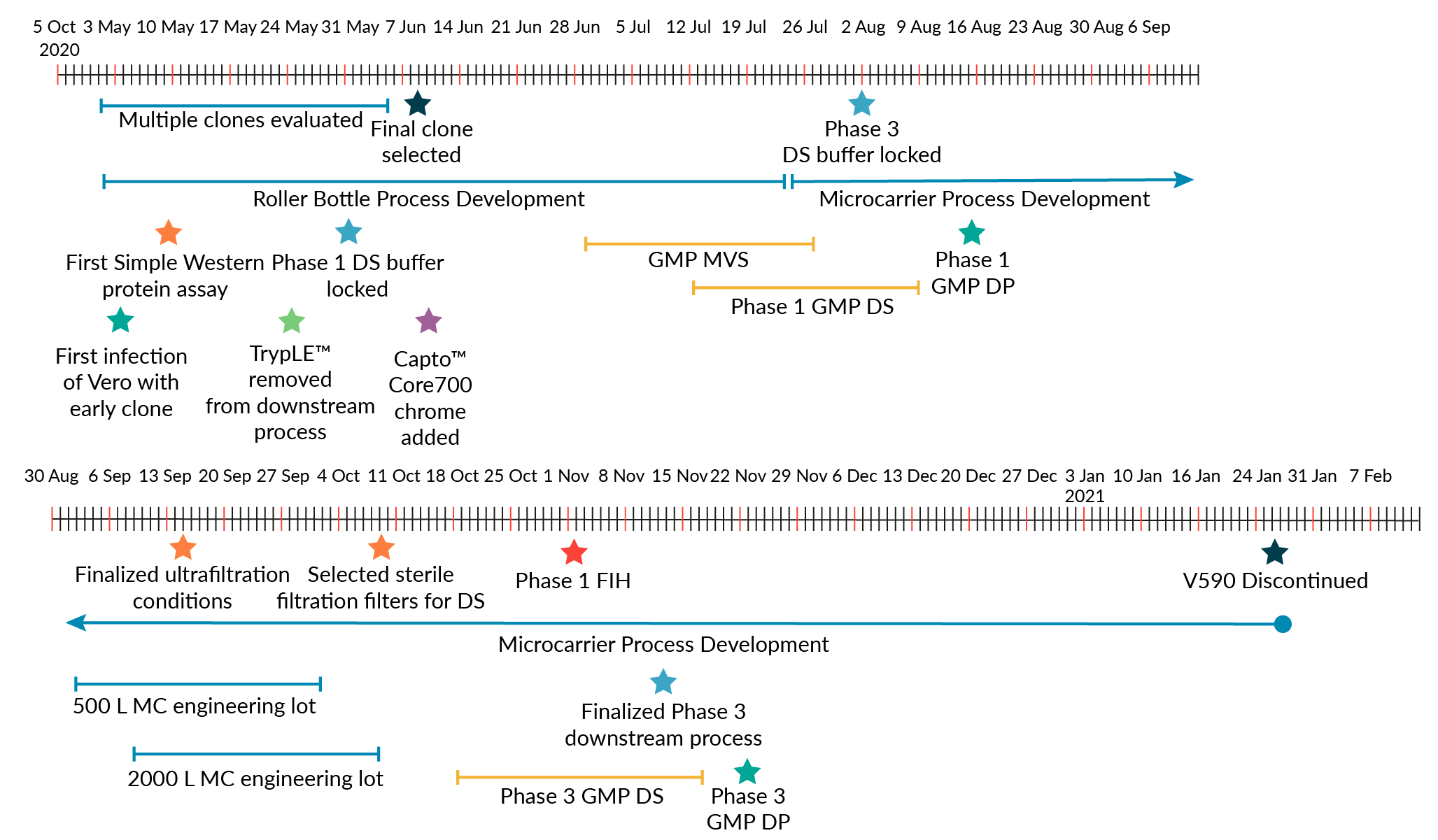 V590 development timeline.DP: Drug product; DS: Drug substance; FIH: First in human; MC: Microcarrier; MVS: Master virus seed.). This is in contrast to traditional preclinical development of vaccines, which usually takes 1−2 years [17]Espeseth AS, Yuan M, Citron M et al. Preclinical immunogenicity and efficacy of a candidate COVID-19 vaccine based on a vesicular stomatitis virus-SARS-CoV-2 chimera. EBioMedicine 2022; 82, 104203. .
V590 development timeline.DP: Drug product; DS: Drug substance; FIH: First in human; MC: Microcarrier; MVS: Master virus seed.). This is in contrast to traditional preclinical development of vaccines, which usually takes 1−2 years [17]Espeseth AS, Yuan M, Citron M et al. Preclinical immunogenicity and efficacy of a candidate COVID-19 vaccine based on a vesicular stomatitis virus-SARS-CoV-2 chimera. EBioMedicine 2022; 82, 104203. .
Other factors besides leveraging the ERVEBO vaccine production platform also enabled V590 process development and production of Phase 1 clinical supplies. The development of multiple Simple Western™ assays allowed for rapid (<1 day) turnaround of analytical results to measure viral and host cell protein levels across downstream processing steps [18]Gillespie PF, Wang Y, Hofmann C et al. Understanding the Spike Protein in COVID-19 Vaccine in Recombinant Vesicular Stomatitis Virus (rVSV) Using Automated Capillary Western Blots. ACS Omega 2023; 8(3), 3319–3328. . On-demand potency with rapid plaque and microplaque assays was also quickly established, providing virus infectivity results in less than 48 hours. Project teams were also highly coordinated to align objectives and experimental plans across several workstreams (upstream, downstream, formulation, analytics).
The development time for the COVID-19 vaccine was also shortened by running development and manufacturing activities in parallel. While Phase 1 clinical materials were being manufactured, development and scale-up activities for the commercial-scale production process were executed at the same time, thus reducing cycle times by approximately 18 months. Cross-training was also implemented to ensure efficient process transfer to clinical production, and manufacturing staff were trained on each process step prior to GMP manufacture. This training provided opportunities for the manufacturing team to develop a strong technical knowledge of the production platform. Both Ebola and COVID-19 vaccine production leveraged existing MSD manufacturing facilities with standard unit operations, which enabled rapid process transfer to the clinical manufacture area. In addition, leveraging the existing rVSV vaccine production platform facilitated rapid scale-up and manufacture by utilizing the available validated equipment and GMP-quality raw materials for manufacturing.
To maintain an accelerated timeline, there was significant pre-investment into the development of the commercial-scale production process prior to the availability of Phase 1 clinical results. For commercial-scale production of V590, it was not possible to leverage the Phase 1 roller bottle process for large-scale manufacture. To achieve the number of vaccine doses required to support the pandemic scale, the commercial manufacture process would need approximately 10,000 roller bottles per batch. Thus, we developed a scalable, microcarrier-based bioreactor (2000 L) production process to generate the number of vaccine doses needed for pandemic scale. The 2000 L bioreactor achieved a peak virus titer of ~1.0e+7 plaque forming unit (PFU)/mL [19]Ton C, Stabile V, Carey E et al. Development and scale-up of rVSV-SARS-CoV-2 vaccine process using single use bioreactor. Biotechnol. Rep. (Amsterdam, Netherlands) 2023; 37, e00782.. The introduction of the bioreactor process also removed an aseptic control risk inherently associated with roller bottle cultures. To this end, the incorporation of terminal sterile filtration was also considered critical to eliminate aseptic processing and reduce the risk of non-sterile product. The use of a microcarrier bioreactor process and the inclusion of a terminal sterile filtration step for the commercial scale V590 production required substantial process development. Several factors enabled commercial-scale process development to be completed quickly:
- The Vero cell expansion process, which is currently used for MSD’s commercial vaccines and part of its LVV microcarrier process, was leveraged for the V590 commercial scale process so that only the microcarrier N-1 cell expansion step had to be developed to supply a sufficient number of cells for 2000 L bioreactor inoculation;
- Experience from the LVV platform process also enabled a consistent and high-quality supply of Vero cells for infection with VSV∆G-SARS-CoV-2 on a regular schedule. This provided material for downstream process development and production of drug substance for assay and formulation development;
- The utilization of single-use technology, existing equipment, consumables, and raw materials enabled process development experiments to start quickly, increased process flexibility, and allowed for rapid implementation of process changes and demonstration of process iterations [19];
- Processing buffers/media were identified early in development, and the number of buffers used was minimized to reduce the workload required for qualification testing;
- The same type of filters for the clarification (Sartoclean® CA, Satorius) and hollow fiber tangential flow filtration (ReadyToFilter Hollow Fiber Cartridge, 750 kilodaltons nominal molecular weight cutoff membrane, Cytiva) steps that were used for the of manufacture Phase 1 supplies were used in the Phase 3 process. Volumetric loadings were optimized to minimize filter surface area requirements.
While LVV development usually takes approximately 18–24 months from clone selection to implementation of a Phase 3 clinical manufacturing process, the factors above enabled MSD to develop a Phase 3 GMP-compliant 2000 L single-use bioreactor process for V590 in approximately 5 months from clone selection, with Phase 3 clinical supply produced in less than 6 months.
Regulatory interactions
Before ERVEBO approval, emergency use doses were provided to Africa using the IND under Expanded Access protocols. Because this vaccine targeted an unmet medical need, ERVEBO was granted Breakthrough Therapy designation by the FDA and PRIority MEdicines (PRIME) designation by the European Medicines Agency (EMA) [20]Wolf J, Bruno S, Eichberg M et al. Applying lessons from the Ebola vaccine experience for SARS-CoV-2 and other epidemic pathogens. NPJ Vaccines 2020; 5(1), 51. . This enabled increased interactions with the regulators (~23 interactions between the EMA and FDA) throughout Biologics License Application and Marketing Authorization Application submission and approval. The applications were submitted using a rolling submission strategy agreed upon with the regulators and also leveraged a collaborative review process with WHO, African VAccine REgulatory Forum (AVAREF), and multiple African countries to ensure approvals were obtained expeditiously where the vaccine was needed most. An overview of expected and actual review periods is shown in Table 1.
| Table 1. Overview of the expected and actual regulatory agency review periods. | |||
| Standard review period | Accelerated review period | ERVEBO® Experience | |
| FDA | 6–10 months | 6 months (Priority Designation) | ~3 months |
| EMA | 210 days (12–14 months to obtain MA) | 150 days (8 months to obtain MA) | ~8 months to obtain Conditional MA |
| WHO prequalification | Median consistently 200 days following reference NRA approval | Shortly following reference NRA approval | 1 day following reference NRA approval |
| Participating NRAs (individual countries participating) | Varies: typically 2–4 years following reference NRA approval | Maximum 90 days following reference NRA approval (per roadmap) | Ongoing: earliest obtained 39 days following reference NRA approval |
| EMA: European Medicines Agency; FDA: Food & Drug Administration; MA: Marketing Application; NRA: National Regulatory Authority; WHO: World Health Organization. | |||
For the COVID-19 vaccine candidate, MSD was able to leverage the ERVEBO roller bottle platform production process to waive preliminary nonclinical studies. Due to the urgency caused by the pandemic, MSD was also able to engage early with agencies to discuss options to accelerate the path to first-in-human. These early engagements included a pre-IND meeting, multiple informal meetings with the FDA and EMA, and Type C written interactions all enabling rapid response and a collaborative sponsor-regulator experience. The early interactions with the FDA enabled the use of a Type V Drug Master File to submit available CMC sections for review earlier than the complete Phase 1 IND package. This allowed the Phase 1 review process to proceed to first-in-human much faster than the normal timeline. After Phase 1, Type C written feedback was also rapidly obtained by submitting written background packages to the IND instead of holding Type C meetings. MSD’s V590 was found to be safe in a Phase 1 clinical trial but was discontinued due to low antibody responses [21]Robbins JA, Tait D, Huang Q et al. Safety and immunogenicity of intramuscular, single-dose V590 (rVSV-SARS-CoV-2 Vaccine) in healthy adults: Results from a phase 1 randomised, double-blind, placebo-controlled, dose-ranging trial. EBioMedicine 2022; 82, 104138..
Expanded access
Prior to ERVEBO approval, the rVSV Ebola investigational vaccine was used to help to contain the outbreaks in the Democratic Republic of the Congo and surrounding countries. Hundreds of thousands of labeled stockpile vaccine doses were deployed through a pre-license access pathway. Pre-license access aims to provide life-saving investigational drugs or vaccines prior to the approval of the drug or vaccine by a regulatory authority. MSD partnered closely with the WHO to align relevant health authority requirements for the use and export/import of rVSV Ebola vaccine into outbreak countries. MSD’s quality control systems were responsible for assessing and approving the WHO’s pre-license access requests and subsequently releasing investigational vaccine lots for use in designated countries. Following release, the MSD logistics organization closely collaborated with specialized pharmaceutical couriers, airlines, and WHO country representatives to seamlessly and routinely deliver vaccine supplies under -70°C dry ice shipment conditions. Extensive pre-license access experience was gained in providing rVSV Ebola vaccine. Lessons learned over time have been employed forward to streamline pre-license access processes and better prepare MSD to respond to future expanded access needs.
Conclusion
MSD’s responses to the Ebola epidemic and SARS-CoV-2 pandemic have demonstrated the benefit of leveraging the rVSV vaccine platform for the rapid development of vaccines. Despite the unprecedented speed of developing these vaccines, opportunities exist for further acceleration of development timelines. As the human population continues to grow and there is habitat destruction, urban development, and increased global travel, the Ebola virus epidemic and SARS-CoV-2 pandemic will not be the last infectious disease outbreaks impacting global human health. Stopping the next emerging pandemic will require utilizing vaccine production platforms and technologies to speed process development and manufacturing scale-up. To ensure a high probability of success for a vaccine candidate in a pandemic, establishing multiple vaccine platforms will be key in developing an effective vaccine quickly. Improvements in regulatory policies and communications to enhance their flexibility without compromising vaccine safety and efficacy are also critical for pandemic preparedness. Long-term strategies for investment into developing new vaccine platforms and application of new technologies for manufacturing infrastructure must be implemented to prepare for accelerated response to future pandemics. Taking these steps for proper preparation will facilitate rapid vaccine development and production to protect society from future public health emergencies.
References
1. Al-Jighefee HT, Najjar H, Ahmed MN, Qush A, Awwad S, Kamareddine L. COVID-19 Vaccine Platforms: Challenges and Safety Contemplations. Vaccines 2021; 9(10), 1196. Crossref
2. Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments [published correction appears in Nat. Rev. Immunol. 2021 Jan 5]. Nat. Rev. Immunol. 2021; 21(2), 83–100. Crossref
3. Scher G, Schnell MJ. Rhabdoviruses as vectors for vaccines and therapeutics. Curr. Opin. Virol. 2020; 44, 169–182. Crossref
4. Rose JK, Schubert M. Rhabdovirus genomes and their products. In: The Rhabdoviruses (Editor: Wagner RR). 1987; 129–166, Plenum Publishing Corporation. Crossref
5. Lawson ND, Stillman EA, Whitt MA, Rose JK. (1995). Recombinant vesicular stomatitis viruses from DNA. Proc. Nat. Acad. Sci. USA 1995; 92(10), 4477–4481. Crossref
6. Whitt MA. Generation of VSV pseudotypes using recombinant ΔG-VSV for studies on virus entry, identification of entry inhibitors, and immune responses to vaccines. J. Virol. Methods 2010; 169(2), 365–374. Crossref
7. Yahalom-Ronen Y, Tamir H, Melamed S et al. A single dose of recombinant VSV-∆G-spike vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020; 11(1), 6402. Crossref
8. Monath TP, Fast PE, Modjarrad K et al.; Brighton Collaboration Viral Vector Vaccines Safety Working Group (V3SWG). rVSVΔG-ZEBOV-GP (also designated V920) recombinant vesicular stomatitis virus pseudotyped with Ebola Zaire Glycoprotein: Standardized template with key considerations for a risk/benefit assessment. Vaccine 2019; 1, 100009. Crossref
9. Yasumura Y, Kawakita Y. Studies on SV40 in tissue culture-preliminary step for cancer research in vitro. Nihon Rinsho. 1963; 21(21), 1201–1215. Crossref
10. Barrett PN, Mundt W, Kistner O, Howard MK. Vero cell platform in vaccine production: moving towards cell culture-based viral vaccines. Exp. Rev. Vaccines 2009; 8(5), 607–618. Crossref
11. Desmyter J, Melnick JL, Rawls WE. Defectiveness of interferon production and of rubella virus interference in a line of African green monkey kidney cells (Vero). J. Virol. 1968; 2(10), 955–961. Crossref
12. World Health Organization (WHO), WHO Statement on the Meeting of the International Health Regulations Emergency Committee Regarding the 2014 Ebola Outbreak in West Africa, 2014. Crossref
13. Wolf J, Jannat R, Dubey S et al. Development of Pandemic Vaccines: ERVEBO Case Study. Vaccines (Basel) 2021; 9(3), 190. Crossref
14. Henao-Restrepo AM, Longini IM, Egger M et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 2015; 386(9996), 857–866. Crossref
15. Henao-Restrepo AM, Camacho A, Longini IM et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!) [published correction appears in Lancet. 2017 Feb 4;389(10068):504] Lancet 2017; 389(10068), 505–518. Crossref
16. Saville M, Cramer JP, Downham M et al. Delivering pandemic vaccines in 100 days – what will it take? N. Engl. J. Med. 2022; 387, e3. Crossref
17. Espeseth AS, Yuan M, Citron M et al. Preclinical immunogenicity and efficacy of a candidate COVID-19 vaccine based on a vesicular stomatitis virus-SARS-CoV-2 chimera. EBioMedicine 2022; 82, 104203. Crossref
18. Gillespie PF, Wang Y, Hofmann C et al. Understanding the Spike Protein in COVID-19 Vaccine in Recombinant Vesicular Stomatitis Virus (rVSV) Using Automated Capillary Western Blots. ACS Omega 2023; 8(3), 3319–3328. Crossref
19. Ton C, Stabile V, Carey E et al. Development and scale-up of rVSV-SARS-CoV-2 vaccine process using single use bioreactor. Biotechnol. Rep. (Amsterdam, Netherlands) 2023; 37, e00782. Crossref
20. Wolf J, Bruno S, Eichberg M et al. Applying lessons from the Ebola vaccine experience for SARS-CoV-2 and other epidemic pathogens. NPJ Vaccines 2020; 5(1), 51. Crossref
21. Robbins JA, Tait D, Huang Q et al. Safety and immunogenicity of intramuscular, single-dose V590 (rVSV-SARS-CoV-2 Vaccine) in healthy adults: Results from a phase 1 randomised, double-blind, placebo-controlled, dose-ranging trial. EBioMedicine 2022; 82, 104138. Crossref
Affiliations
Christopher Ton
Process Research & Development,
Merck & Co., Inc., Rahway, NJ, USA
Michael A Winters
Process Research & Development,
Merck & Co., Inc., Rahway, NJ, USA
Raymond Ducoat
Process Research & Development,
Merck & Co., Inc., Rahway, NJ, USA
Douglas D Richardson
Analytical Research & Development,
Merck & Co., Inc., Rahway, NJ, USA
Kristine Fuller
Chemistry, Manufacturing, and Controls, Merck & Co., Inc., Rahway, NJ, USA
Melissa Hughes
Global Clinical Supply, Merck & Co., Inc., Rahway, NJ, USA
Authorship & conflict of interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: The authors acknowledge Tara Tagmyer and Kay Hunsberger for their assistance in preparing this manuscript.
Disclosure and potential conflicts of interest:The authors have no conflicts of interest.
Funding declaration: Development of ERVEBO® was funded in whole or in part with Federal Funds from the Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority under Contract Number: HHSO100201500002C. The V590 program has been funded in part with Federal funds from the Department of Health and Human Services, Administration for Strategic Preparedness and Response; Biomedical Advanced Research and Development Authority (BARDA), under Contract No. HHSO100201600031C.
Article & copyright information
Copyright: Published by Vaccine Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2023 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. Published by Vaccine Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Mar 29 2023; Revised manuscript received: May 24 2023; Publication date: Jun 14 2023.
