Vaccine manufacturing & analytics: INFOGRAPHIC
Published: 16 July 2024
Infographic
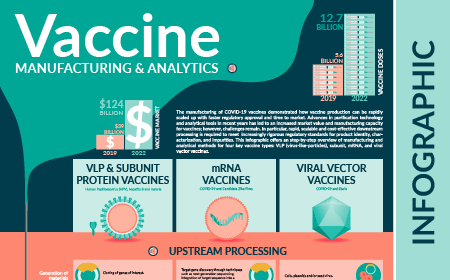 | The manufacturing of COVID-19 vaccines demonstrated how vaccine production can be rapidly scaled up with faster regulatory approval and time to market. Advances in purification technology and analytical tools in recent years has led to an increased market value and manufacturing capacity for vaccines; however, challenges remain. In particular, rapid, scalable and cost-effective downstream processing is required to meet increasingly rigorous regulatory standards for product identity, characterization, and impurities. This infographic offers a step-by-step overview of manufacturing and analytical methods for four key vaccine types: VLP (virus-like-particle), subunit, mRNA, and viral vector vaccines. |
