Immunity and vaccine development against liver-stage malaria
Vaccine Insights 2024; 3(5), 143–162
DOI: 10.18609/vac.2024.026
Malaria is still a major cause of death, with over half a million people dying every year and over 200 million infections. As of 2024, two protein subunit vaccines have gained WHO approval, the RTS,S/AS01 and R21/Matrix-M, with 30% and 75% vaccine efficacy, respectively. Despite these significant milestones, readiness for global implementation, and the potential to drastically reduce the global disease burden from malaria, there are limitations in the current approaches. This is in part due to logistical and scalability issues of the complicated subunit vaccine but also the low efficacy and requirement for 3–4 doses and a booster dose prior to the malaria season every year to maintain efficacy. Furthermore, the WHO has set the target of 90% vaccine efficacy over 12 months for future malaria vaccine development. This review will highlight the limitations to the current approaches of generating antibodies against the NANP domain of circumsporozoite protein, and the need for new approaches and technologies. Specifically, we will discuss vaccines generating the newly identified liver tissue-resident memory CD8 T cell that can provide sterile protection against liver-stage infection. Live-attenuated, protein subunit, viral vector and mRNA vaccines are considered in this review. Since the COVID-19 pandemic, mRNA vaccines have demonstrated rapid and wide-spread deployment that may overcome logistical hurdles faced by other vaccines. mRNA vaccines could be harnessed for improved generation of liver tissue-resident memory T cells and improved, long-lasting malaria immunity.
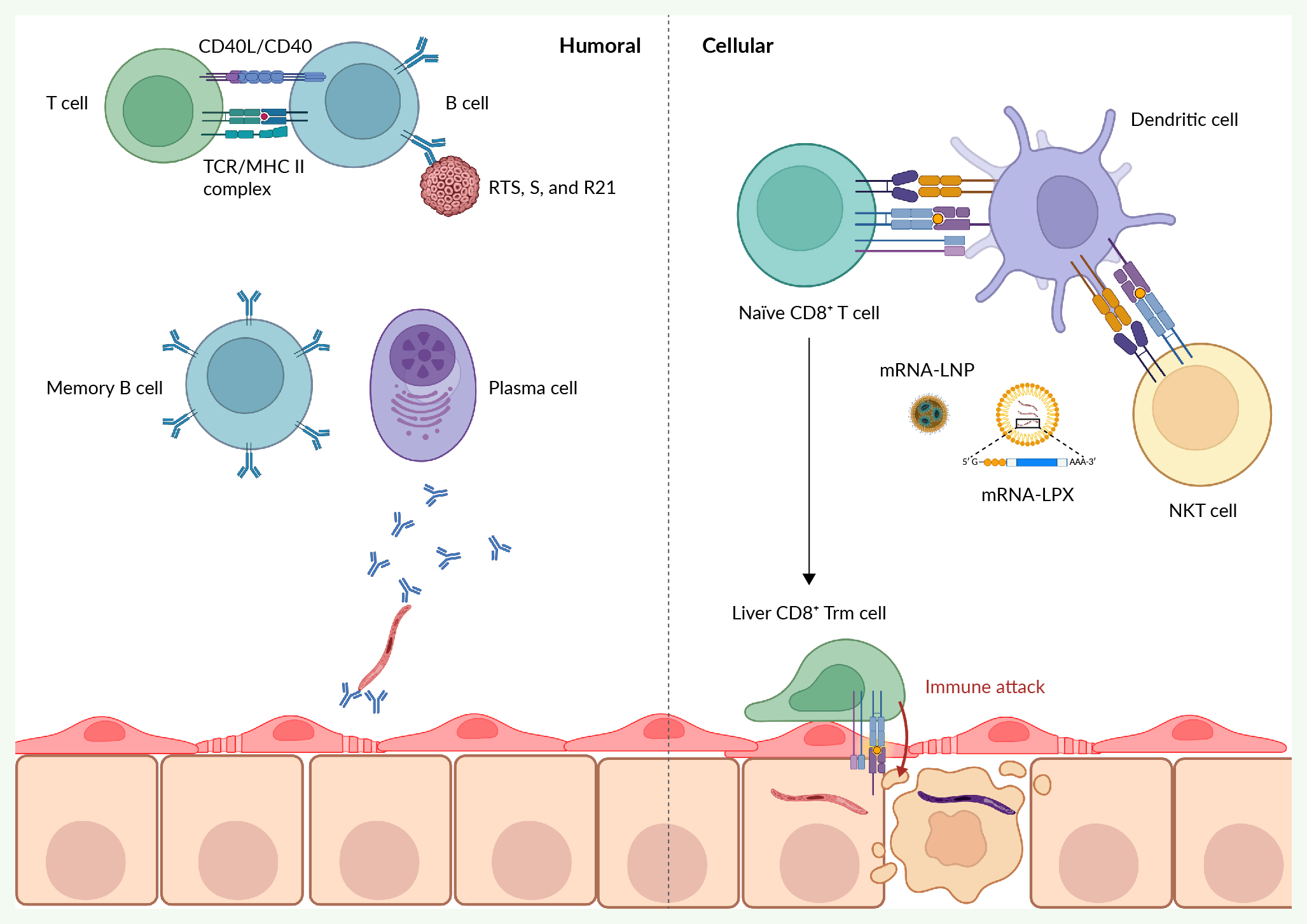
Created with Biorender®.