Aug
30
2023
On demand
Streamlining vaccine development and production with modality-specific purification tools for mRNA and beyond
Wednesday 08:00 PDT / 11:00 EDT / 16:00 BST / 17:00 CEST
Many factors contributed to the impressive speed with which COVID-19 vaccines were created, produced, and deployed, including novel vaccine modalities and recent advancements in downstream purification tools. These advancements remain powerful in the continued search for vaccines against persistent infectious agents such as HIV and malaria.
This webinar will present improved purification tools developed for rapid candidate screening and intensified downstream processing of various vaccine modalities, including mRNA, viral vector vaccines, and virus-like particles (VLPs). Examples of each vaccine modality and the associated purification tools will be given, including case studies and performance data.
Attend this webinar to learn more about
- The variety of vaccine modalities used in the COVID-19 response and potential strategies for future vaccine development
- Easy vaccine candidate screening via C-tag technology and its advantages over His-Tag
- How affinity chromatography resins can help to improve the purification process of new vaccine modalities such as mRNA- or protein-based vaccines or viral vectors
- How ion exchange resins can be leveraged for purifying VLPs and viral vector vaccines
You might also like
Combining AI-powered mRNA design with non-viral delivery to achieve organ-specific targeting
Claire Guéguen, Davide De Lucrezia
15 July
in 4
Days

Enhancing mRNA production with advanced analytics: WACKER Biotech
Michael Daume, Blaz Bakalar
8 October 2024
Watch
Quality by design in mRNA manufacturing: at-line monitoring coupled with DOE for higher IVT yield and efficiency of purification
Rok Sekirnik
3 December 2024
Watch
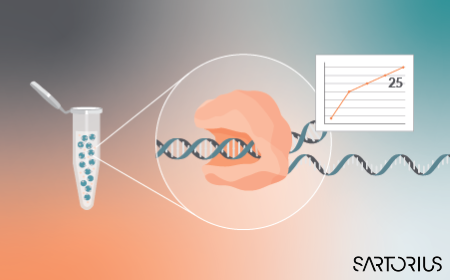
Optimizing mRNA production: achieving 25 g/L mRNA yield with low dsRNA by integrating PAT
Rok Sekirnik
8 May
Watch
Optimizing plasmid DNA purification: strategies for reliable and expandable processes
Christy Franco, Jasmina Puc
17 June
Watch

