Cell & Gene Therapy Insights is an online, open access, peer-reviewed journal with an interdisciplinary, translational focus on all aspects of the cell and gene therapy field.
Cell & Gene Therapy Insights addresses the important challenges and advances in advanced therapies, publishing original research, reviews, commentary articles, clinical trial reports, and much more. You can find more information about our aims and scope here.
Sign-up for free for unlimited access to all of our articles, webinars, podcasts, news, interviews, and more.
April 2025
Upcoming webinars
To 1000L and beyond: streamlining AAV manufacturing with transfection complex stabilization
Leisha Kopp, Mason Bonitz
3 June
in 5
Days
Optimizing for faster quality control in cell therapies: leveraging rapid detection methods
Srinath Kashi Ranganath
5 June
in 7
Days
Leveraging fluid dynamics characterization to accelerate cell therapy product development
Hamza Patel, Tom Heathman
10 June
in 12
Days

AAV production and purification: key steps from design to GMP readiness
G Ganjam, F Leseigneur, E Jackson-Holmes
11 June
in 13
Days

Non-viral GMP manufacturing for engineered cells at clinical and commercial scale
Leif Anderson, Chris Abraham
12 June
in 14
Days

Combining CRISPR and transposon-based technologies for improved sdAb-based CAR-T therapies
Begoña Diez Cabezas, Juan Roberto Rodriguez-Madoz
17 June
in 19
Days
Latest Articles
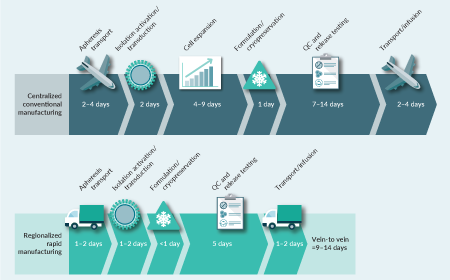
Full speed ahead: how rapid CAR-T manufacturing can shape the cell therapy landscape
Mackenzie Lieberman, Kathryn Henckels
29 May

Why choose a modular, automated approach to autologous CAR T manufacturing?: INFOGRAPHIC
28 May

Revolutionizing non-viral gene delivery with silicon-stabilized LNPs
Suzanne Saffie-Siebert
20 May

Efficient AAV purification: resin reuse and scalable polish method
Duncan Dulac
19 May

Optimizing upstream processing to improve consistency and scalability in viral vector production
Katerina Rigaki
19 May

Next-gen immunotherapy: a guide for CGT developers
Alex Sargent
9 May
Exploring innovations in AAV upstream processing to advance gene therapy development
Hao Liu
8 May

Register for Bioconjugation Insights
7 May

Essential equipment for advancing cell and gene therapy: innovations in incubation, monitoring, and cold storage
Tia Harmon
7 May