Filter by Interests
Filter by ContentType

Illuminating pathways and balancing precision: maximizing economic value in breast cancer gene therapy through integrated CMC, analytics, and collaboration
Arya Bhushan , Preeti Misra
10 September 2024
Commentary
Combining best practices and analytical reference materials to tackle challenges in AAV manufacturing
Mark Verdecia
03 April 2024
Commentary
Key considerations & challenges in developing flow cytometry assays for cell & gene therapy products
Ajit Kamath
12 December 2022
Commentary
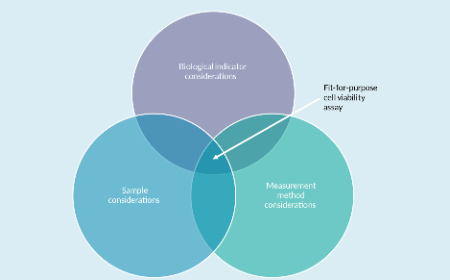
Outcomes from a cell viability workshop: fit-for-purpose considerations for cell viability measurements for cellular therapeutic products
L Pierce, S Sarkar, L Li-Ying Chan et al
14 May 2021
Commentary
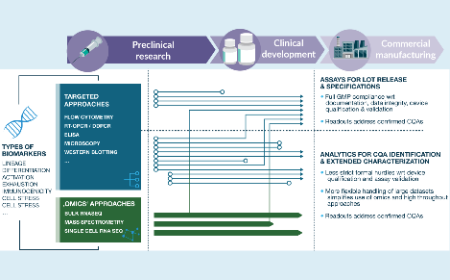
Finding quality in complexity: how cellular therapeutics are shifting analytical paradigms for clinical supply and product manufacturing
Vilma Jimenez Sabinina, Dominic Günter Hildebrand
13 May 2021
Commentary

Master ATMP processes by controlling raw and starting material
Anne-Sophie Lebrun, Carmen Brenner
24 March 2021
Commentary

Considerations for performing virtual quality audits on manufacturers of gene therapy viral vectors: an auditor’s perspective during the COVID-19 public health emergency
Gary C du Moulin, PhD, MPH, RAC
18 March 2021
Commentary

The right analytical toolbox is key to moving your pipeline forward
Andrew Espejo
25 August 2020
Commentary
Analytical Technology Used in the Latest Development of Gene Therapy Candidates
Z Li, Z Wu, T Maekawa et al
16 May 2019
Commentary