Filter by Interests
Filter by ContentType

Navigating the EU joint clinical assessment process: key considerations for manufacturers of ATMPs and oncology medicines
Clare L Hague
18 June 2024
Commentary
Combining best practices and analytical reference materials to tackle challenges in AAV manufacturing
Mark Verdecia
03 April 2024
Commentary
Unravelling immunogenicity induced by Cas9 in gene therapy: a comprehensive commentary of current understanding and implications
Anna Lina Cavallo
08 November 2023
Commentary
Overcoming challenges to CAR-T cell therapies in India
A Uppal, R Chakrabarti, N Chirmule et al
08 March 2023
Commentary
Regulatory & supply chain implications for plasmids as critical starting materials in the manufacture of viral vector gene therapy products
M Gosse, C Jones, D Jesus et al
08 March 2022
Commentary
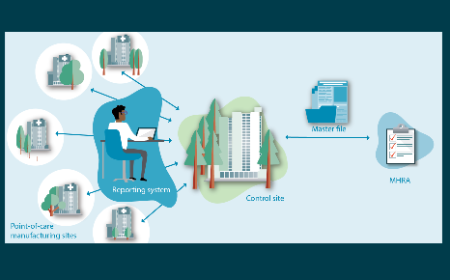
The UK’s emerging regulatory framework for point-of-care manufacture: insights from a workshop on advanced therapies
E Bicudo, I Brass, P Carmichael et al
30 September 2021
Commentary
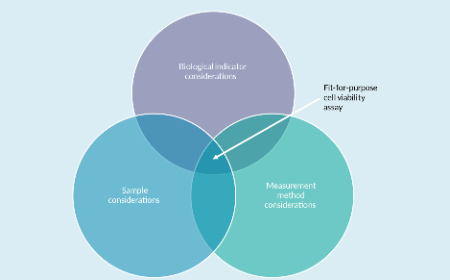
Outcomes from a cell viability workshop: fit-for-purpose considerations for cell viability measurements for cellular therapeutic products
L Pierce, S Sarkar, L Li-Ying Chan et al
14 May 2021
Commentary
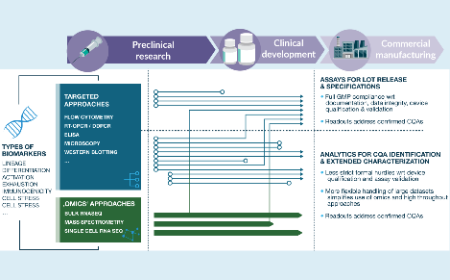
Finding quality in complexity: how cellular therapeutics are shifting analytical paradigms for clinical supply and product manufacturing
Vilma Jimenez Sabinina, Dominic Günter Hildebrand
13 May 2021
Commentary

Considerations for performing virtual quality audits on manufacturers of gene therapy viral vectors: an auditor’s perspective during the COVID-19 public health emergency
Gary C du Moulin, PhD, MPH, RAC
18 March 2021
Commentary