Filter by Interests
Filter by ContentType

‘Winning’ target product profiles for CAR-T cell therapies in oncology: critical success factors for commercially viable therapies
Clare Hague, Louise Street-Docherty, Frances Pearson
19 December 2024
Expert Insight
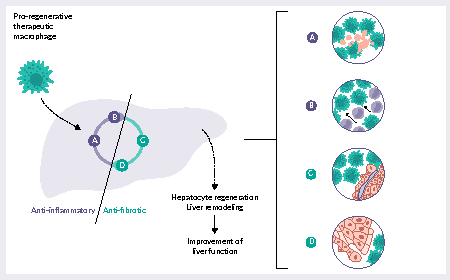
Interpreting the new FDA draft potency guidance: an RNA cell therapy perspective
Damian Marshall, Kayleigh Thirlwell
02 December 2024
Expert Insight

Upstream processing of viral vectors: a summary
Pardhasaradhi Mathi
29 July 2024
Expert Insight

MHRA regulatory considerations for the quality of mRNA products
Ka-Wai Wan, Francis Galaway
22 May 2024
Expert Insight
Raw materials and supplies for cell therapies: end to end expectations and best practices
Lili Belcastro
15 May 2024
Expert Insight
Innovation in hematopoietic stem cell cryopreservation and cold chain management
M Prisciandaro, M Santodirocco, G Fania et al
30 April 2024
Expert Insight
Preclinical trends in developing genetically altered cell therapies
Mary Ellen Cosenza
25 March 2024
Expert Insight
Considerations for development of gene-edited PSC-based therapies
Brent Morse, Amanda Mack
14 November 2023
Expert Insight
Optimizing ddPCR assay for characterizing AAV vector genome integrity
Aishwarya Shevade, John S Reeves, Andrew D Tustian
03 August 2023
Expert Insight
Navigating regulations to provide ethically sourced cellular material for research and development: UK perspective
Salmah Ahmed
11 January 2022
Expert Insight
The Chemistry Manufacturing and Controls (CMC) section of gene therapy-based INDs: overview in a changing landscape
Gabriela Denning, PhD
18 October 2021
Expert Insight
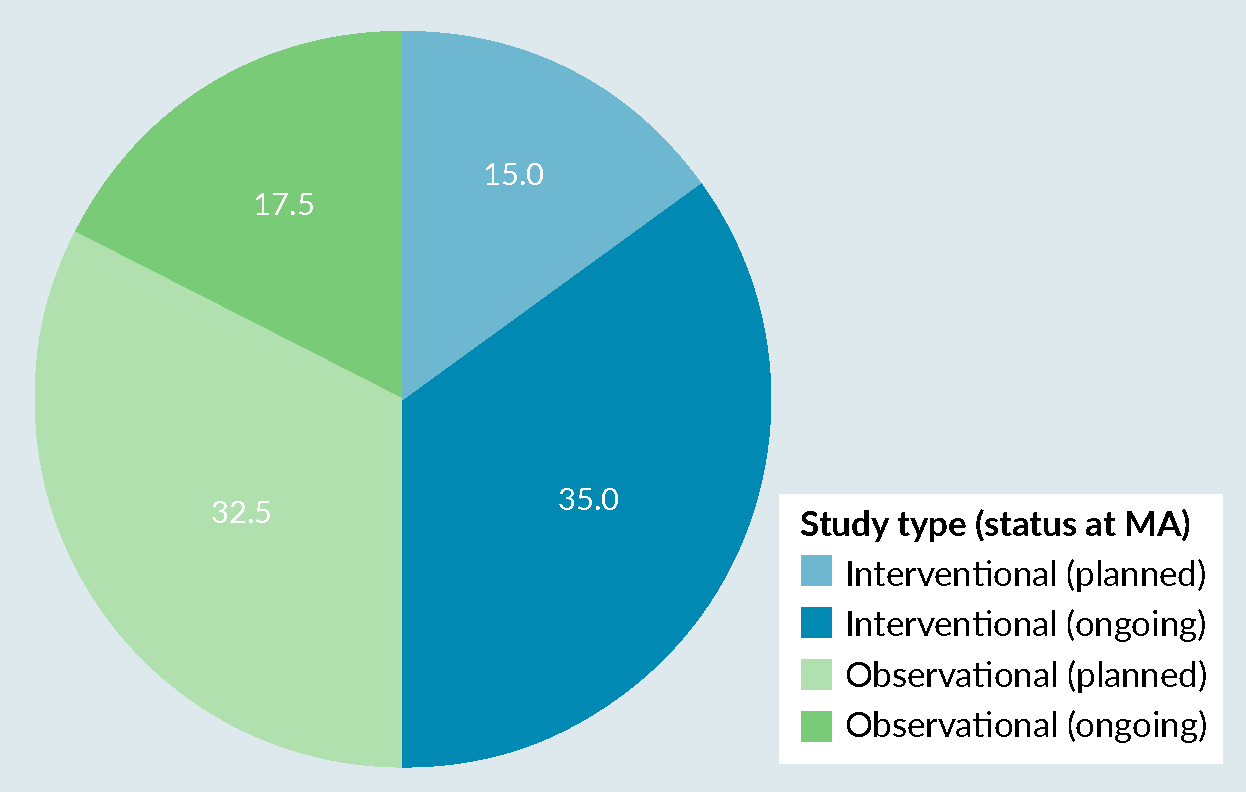
Post-marketing safety and efficacy surveillance of cell and gene therapies in the EU: A critical review
E Fritsche, M Elsallab, M Schaden et al
18 November 2019
Expert Insight
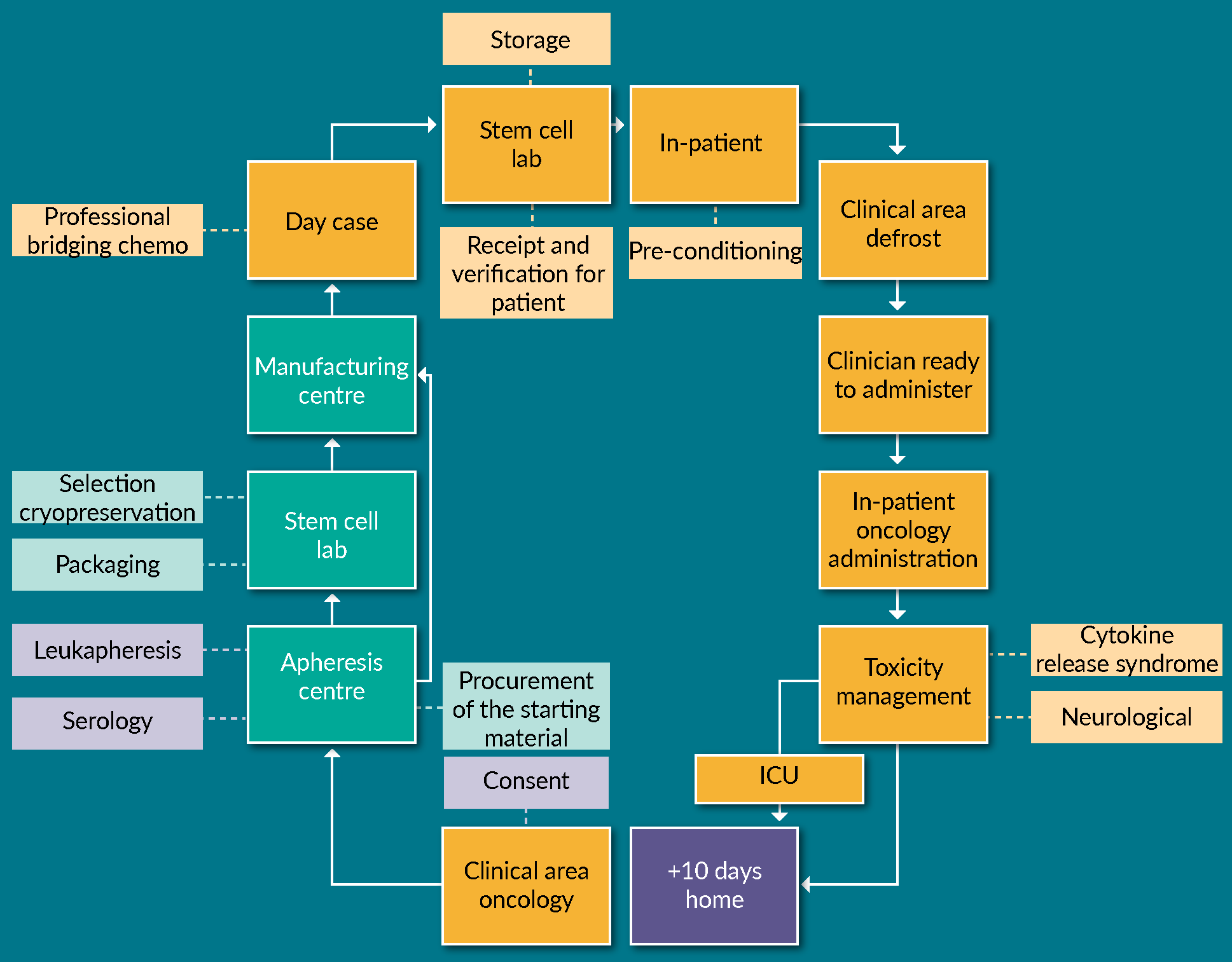
Implementation of advanced therapy medicinal products: a UK pharmacist’s perspective
Anne Black
18 October 2019
Expert Insight
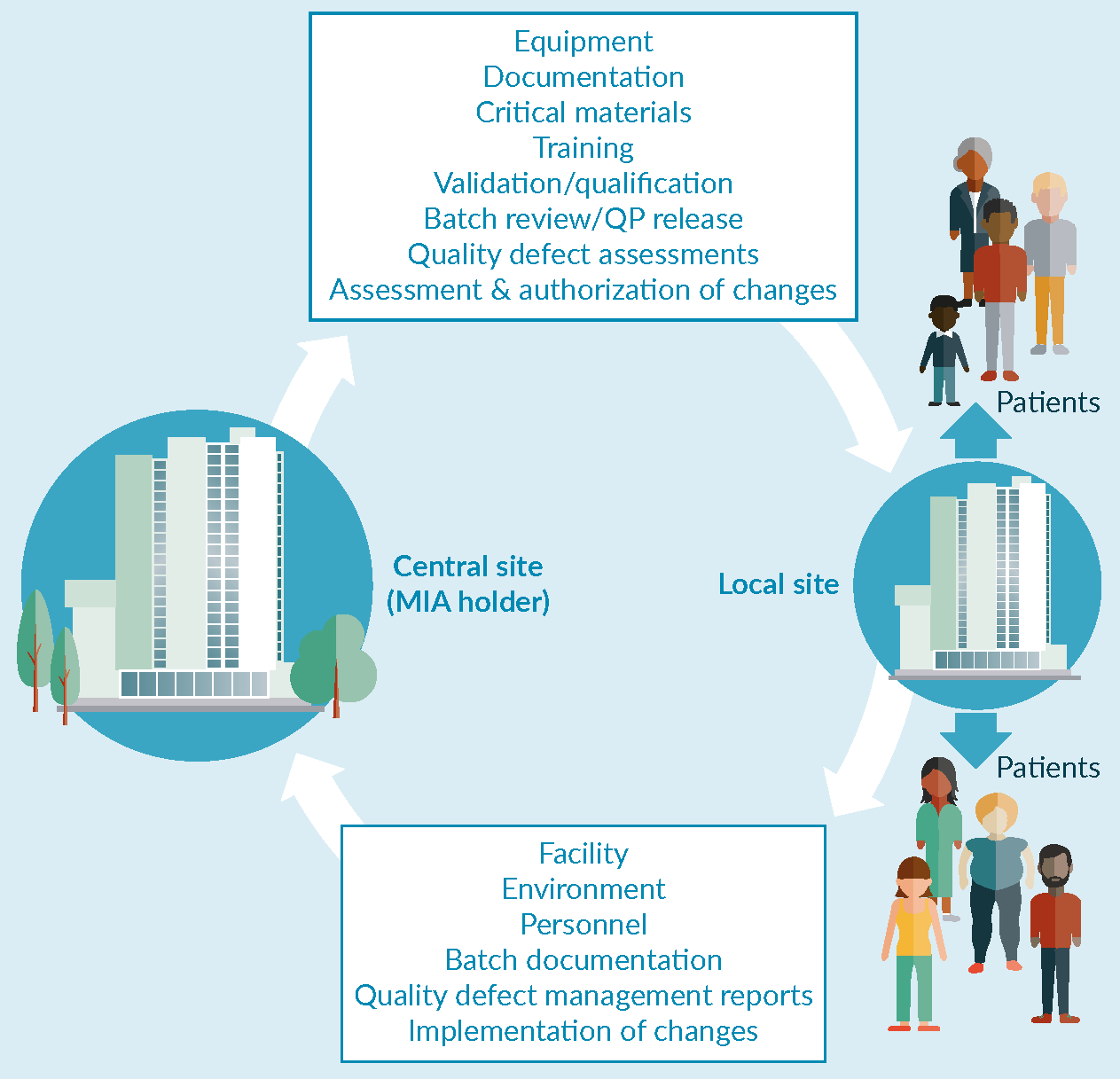
Regulatory considerations for decentralized manufacture of ATMPs
Alison Wilson, Alexis Cockroft
02 October 2019
Expert Insight
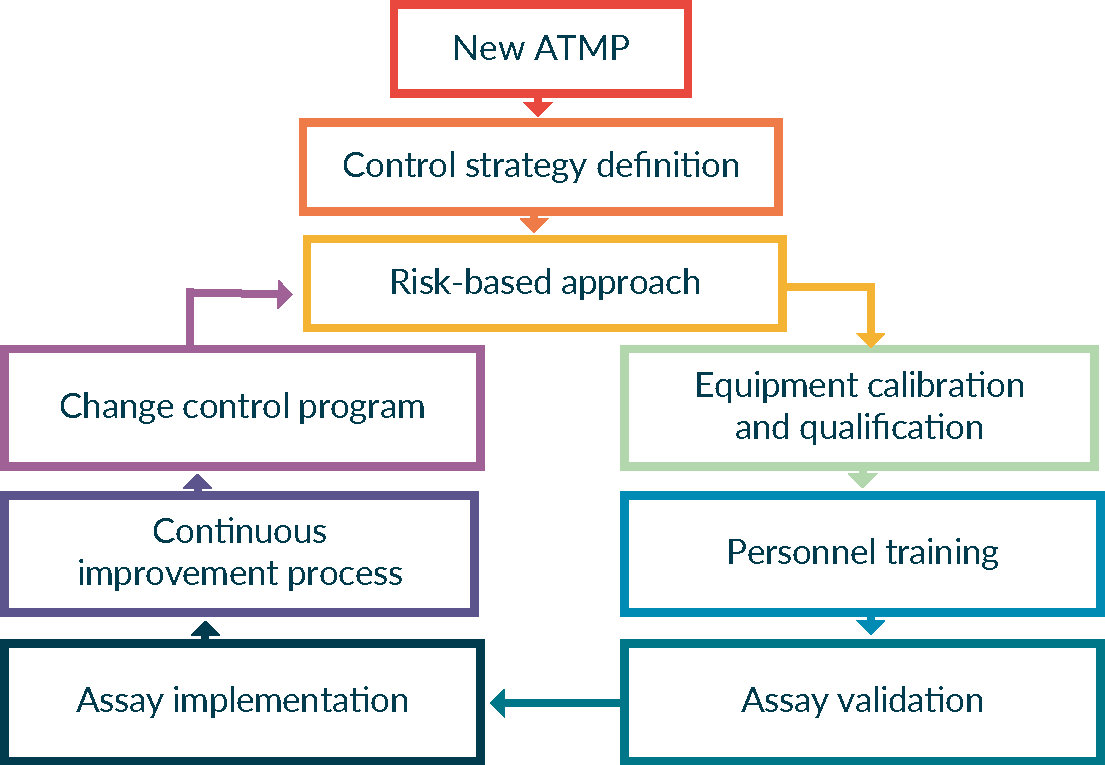
Developing a quality control program for advanced therapy medicinal products in Europe
Margarita Codinach
02 October 2019
Expert Insight
Addressing challenges in meeting chemistry, manufacturing and control regulatory requirements for gene therapy products
Karen Magers
24 July 2019
Expert Insight
Management of ‘out of specification’ commercial autologous CAR-T cell products
Alexey Bersenev, Sven Kili
17 December 2018
Expert Insight

Accelerated Access of Advanced Regenerative Therapies: an Industry Perspective
Yaron Ramati
06 August 2018
Expert Insight
The Regulatory Environment for Cell Therapies in Australia – an Opportunity to Expedite Clinical Development
Simon Bishop, Simone Flight, Natalie Thomas
30 July 2018
Expert Insight

Breakthrough Therapy Designation & Regenerative Medicine Advanced Therapy Designation Programs in Cellular & Gene Therapies
Xiaofei Wang, Wilson W Bryan, Lei Xu
30 July 2018
Expert Insight
Proposed Solutions to Further Improve the Regulatory Landscape for ATMPs in Europe
A Hubert, J Barry, C Vieira et al
17 July 2018
Expert Insight