Filter by Interests
Filter by ContentType
Operations strategy for a scalable CGT supply chain: key CXO insights
Shesh Sharma, Tim Sirichoke, Edward Ballesteros
02 December 2024
Expert Insight
The sweet cell of success: key considerations for the sourcing and production of pluripotent cell lines for therapeutic development
Samuel JI Blackford, Nathan C Manley
21 May 2024
Expert Insight
Raw materials and supplies for cell therapies: end to end expectations and best practices
Lili Belcastro
15 May 2024
Expert Insight
Innovation in hematopoietic stem cell cryopreservation and cold chain management
M Prisciandaro, M Santodirocco, G Fania et al
30 April 2024
Expert Insight

A straightforward tool for developing a raw material supply strategy for cell & gene therapies
K Kulenkampff, R Eigenmann, D Karlstetter et al
08 January 2024
Expert Insight
Considerations for development of gene-edited PSC-based therapies
Brent Morse, Amanda Mack
14 November 2023
Expert Insight
The supporting role of plasmids in gene & cell therapy
Duarte Prazeres
06 July 2023
Expert Insight
Challenges in obtaining cellular therapy starting material for patients with sickle cell disease
Yvette C Tanhehco
22 March 2023
Expert Insight
Taking lessons from nature to improve cell therapy cryopreservation
Jason Acker, Nishaka William, Mackenzie Coatham
20 March 2023
Expert Insight
Implementing demand & operations planning in clinical cell & gene therapy
Peter Horton
26 January 2023
Expert Insight
Considerations for use of hematopoietic stem cells in allogeneic therapies
Barbara Seymour
08 March 2022
Expert Insight

Banking on the future of regenerative medicine with cGMP-compliant iPSC lines
Melissa K Carpenter
22 February 2022
Expert Insight
Umbilical cord blood NK cells offer multiple advantages for cancer immunotherapy: lessons learned from Glycostem’s orphan drug oNKord®
Volker Huppert
14 January 2022
Expert Insight
Navigating regulations to provide ethically sourced cellular material for research and development: UK perspective
Salmah Ahmed
11 January 2022
Expert Insight
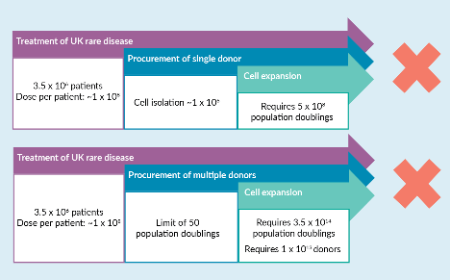
The case for the use of pooled donors in the manufacture of allogeneic cell therapies
Benjamin Weil, Mark Lowdell
24 March 2021
Expert Insight
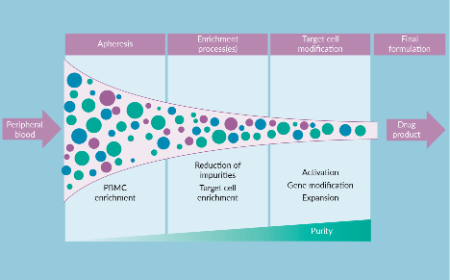
Limiting variability to achieve reproducibility in cell manufacturing
Anne Lamontagne, Andrew Fesnak
11 November 2020
Expert Insight
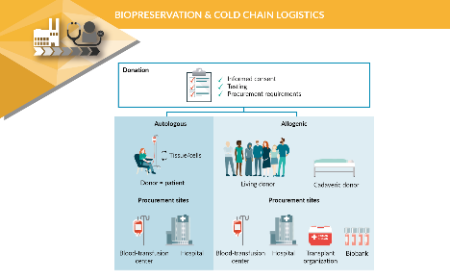
The supply chain: key considerations for biological starting material for ATMPs production
Elisabet Aguilar
22 September 2020
Expert Insight

Using serum-free media to streamline and optimize CAR T-cell manufacturing workflows
Chengkang (CK) Zhang, Amber Jones
10 September 2020
Expert Insight
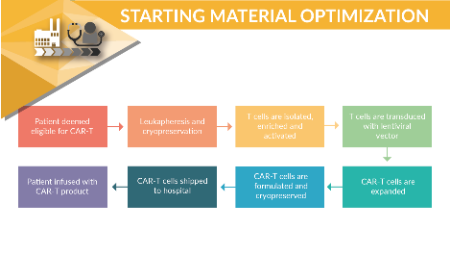
Utilization of risk-based approach to characterize starting material for autologous CAR-T manufacturing
Jean Stanton
08 September 2020
Expert Insight
Supply chain challenges and issues facing the autologous cell manufacturing industry
K Wang, B Wang, C White et al
24 April 2020
Expert Insight
Developing and implementing a supply chain management system for cellular therapy programs
David L DiGiusto, Rakib Ouro-Djobo, Uzair Rajput
09 April 2020
Expert Insight