Filter by Interests
Filter by ContentType
The parent–child IND approach: an interpretation of FDA’s guidance on studying multiple versions of a cellular or gene therapy product in an early-phase clinical trial
Timmothy Taps
03 January 2023
Perspective

Demonstrating Comparability of AAV Gene Therapy Products During Clinical Development: Managing the Link Between the Product and the Process
Niamh Kinsella, Clare Blue
16 December 2021
Perspective
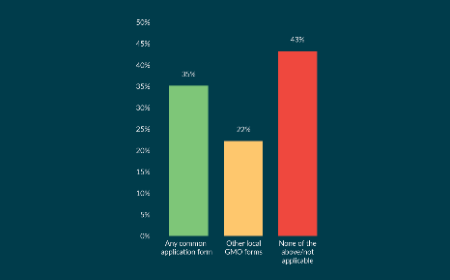
Clinical trials with investigational medicinal products consisting of or containing genetically modified organisms: implementation of Clinical Trials Regulation EU 536/2014
N Lambot, J Awigena-Cook, T Reimer et al
29 September 2021
Perspective
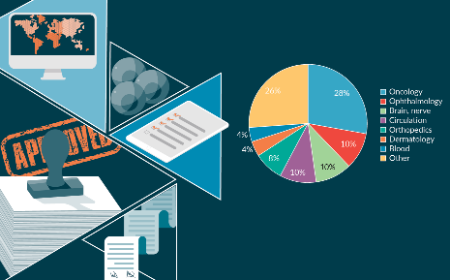
Current status and future perspective of gene therapy products in Japan
Y Maruyama, A Sakurai, M Kasai et al
11 March 2021
Perspective
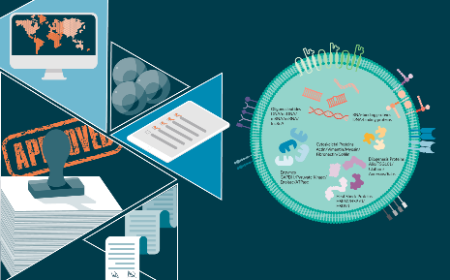
Exosomes as therapeutics and drug delivery vehicles: global regulatory perspectives
Shaun Stapleton
17 December 2020
Perspective

Advanced therapy regulation in the UK: what might the future hold post-Brexit?
Patrick Ginty
18 September 2020
Perspective

Regulatory perspective on ATMPs: device combinations
Ilona Reischl, Stefan Strasser
13 August 2020
Perspective
Regulatory considerations in the development of gene therapies for neurological disorders in the EU region: an industry perspective
S Bennett, L Oliva, S Beattie et al
30 July 2020
Perspective

Clinical development of ATMPs: hospitals as an exemption?
Martin Hildebrandt
29 November 2019
Perspective

Health Technology Assessment of Gene Therapies in Europe and the USA: Analysis and Future Considerations
T Qiu, E Hanna, M Dabbous et al
04 September 2019
Perspective

The diversity in regenerative medicines regulations in Europe, USA and Japan
T Qiu, M Dabbous, L Chachoua et al
04 September 2019
Perspective
Should cell- and gene-therapy developers engage with regulatory authorities?
James W McBlane
22 July 2019
Perspective
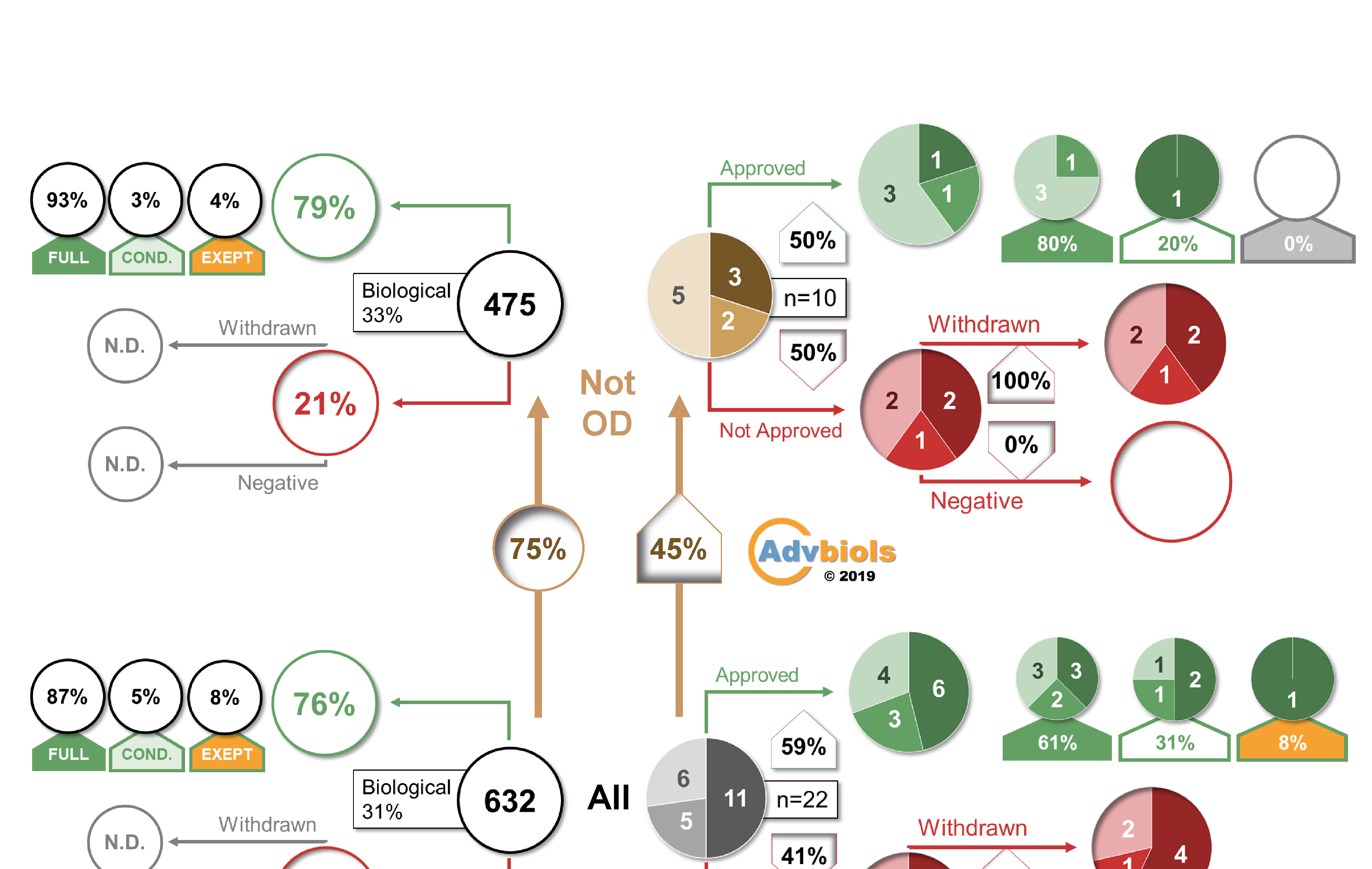
EU market authorisation strategy: lessons from the first 22 ATMP submitted to the EMA
Oliver Ball, Sarah Robinson, Christopher Bravery
25 June 2019
Perspective

Facilities for the future: design & qualification of aseptic manufacturing facilities for cell and gene therapy
Neil WA McGowan, John DM Campbell
25 June 2019
Perspective
Gene therapy success: how to keep its promises and improve – a regulatory perspective
Filomena Nappi, Francesca Capone, Maria Cristina Galli
24 June 2019
Perspective
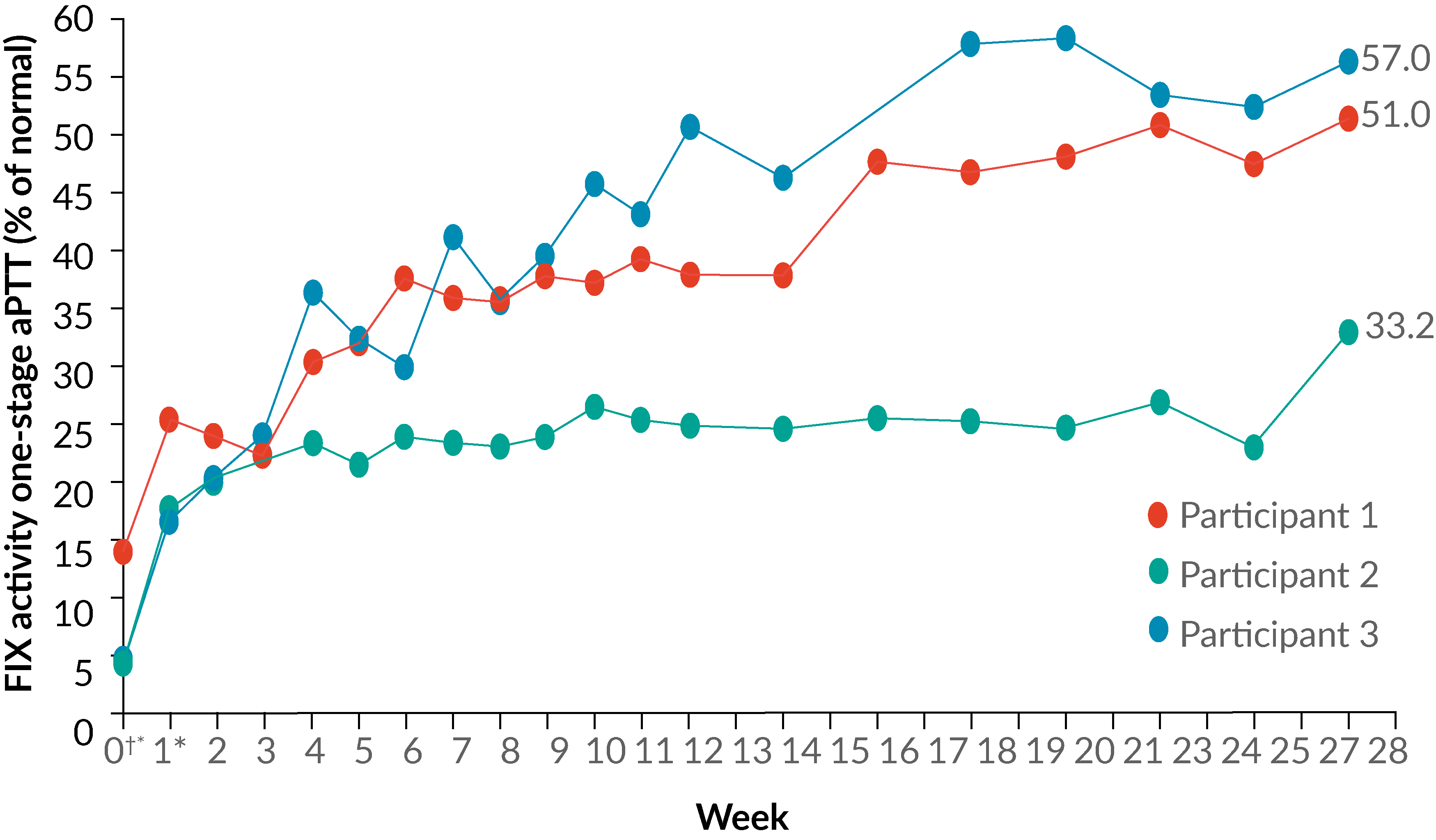
The impact of regulatory strategy on business goals: uniQure’s Phase 3 product optimization for hemophilia B
A Kuta, E Sawyer, M Cantor et al
24 June 2019
Perspective