Filter by Interests
Filter by ContentType
Trends in innovation for enhanced gene therapy downstream process intensification and product yield
Rachel Legmann
28 October 2024
Poster

Navigating evolving regulatory CMC guidance in the AAV gene therapy field
M Brewer, A Cockroft, C Fuentes et al
24 August 2023
Poster

Driving the expansion of mRNA into the therapeutic sphere
Alejandro Becerra, Andreas Kuhn, Metin Kurtoglu
13 July 2023
Poster
Optimizing vector production & purification to enhance scalable AAV manufacturing
Jenny England
24 January 2023
Poster

Regulatory requirements and product attributes for cGMP viral vector production
Scott Cross, Shikha Mishra
19 December 2022
Poster

Manufacturing considerations underpinning viral & non-viral platform selection
A Hagerman, A Noyes, V Slepushkin et al
15 December 2022
Poster

Successful suspension-based viral vector manufacturing scale-up from process development to clinic
D Kole, T P Cripe, L Giannunzio et al
07 December 2022
Poster

How can we maximize the efficiency of large-scale LV vector production?
04 August 2022
Poster

Current technological trends & advancements in vector purification
Y Cai, N Clement, C Gaskin et al
01 July 2022
Poster
Applications and benefits of the CTS Rotea Counterflow Centrifugation System in cell therapy workflows
Carl Dargitz
11 May 2022
Poster
Supply chain efficiency and sourcing for scaling your gene therapy operation
Céline Martin, Don Young
03 May 2022
Poster

Manufacturing of RCA-free adenoviral vectors
Nico Scheer
27 March 2022
Poster

Roundtable Roundup: strategies for scaling up and out in gene therapy manufacturing - addressing AAV’s growing pains
Christopher Reardon, Phillip Vermilion, John Yoshi Shyu
22 March 2022
Poster
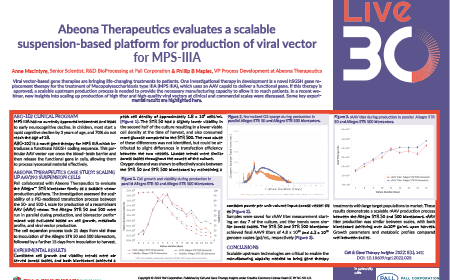
Abeona Therapeutics evaluates a scalable suspension-based platform for production of viral vector for MPS-IIIA
Anne MacIntyre, Phil Maples
17 February 2022
Poster
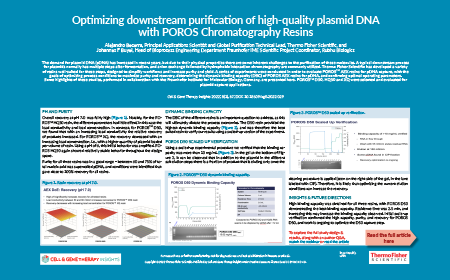
Optimizing downstream purification of high-quality plasmid DNA with POROS™ Chromatography Resins
Alejandro Becerra, Johannes Buyel
14 February 2022
Poster

Advances in AAV process development
Alejandro Becerra, Matthias Hebben, Michael Mercaldi
14 February 2022
Poster

COVID-19 vaccine approvals: key lessons for mRNA therapeutics
V Indurthi, PhD, J Barberio, S Zobbi et al
06 December 2021
Poster
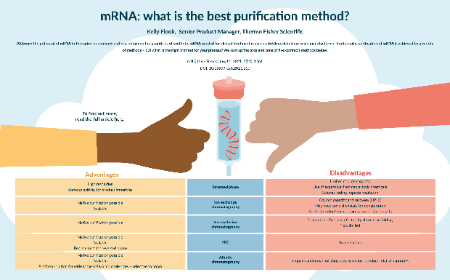
mRNA: what is the best purification method?
Kelly Flook
02 December 2021
Poster