Filter by Interests
Filter by ContentType

Current global regulatory landscape for biodistribution & shedding assessment of rAAV gene therapies & recommendations of the IMI ARDAT consortium on future directions
N Anne Schmidt, J Giblin, T K MacLachlan et al
25 April 2022
Regulatory Insight

The importance of starting materials: quality and regulatory considerations for cell-based therapies
B Bonamassa, P Gasparini, G Pompilio et al
25 March 2021
Regulatory Insight
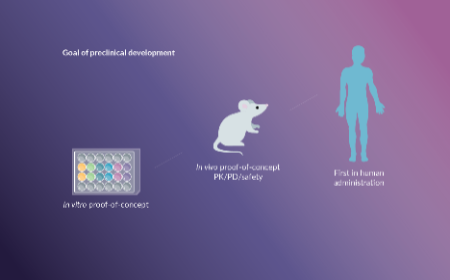
FDA perspective on the preclinical development of cell-based immunotherapies
Alyssa Kosmides Galaro, Christopher Saeui
23 October 2020
Regulatory Insight
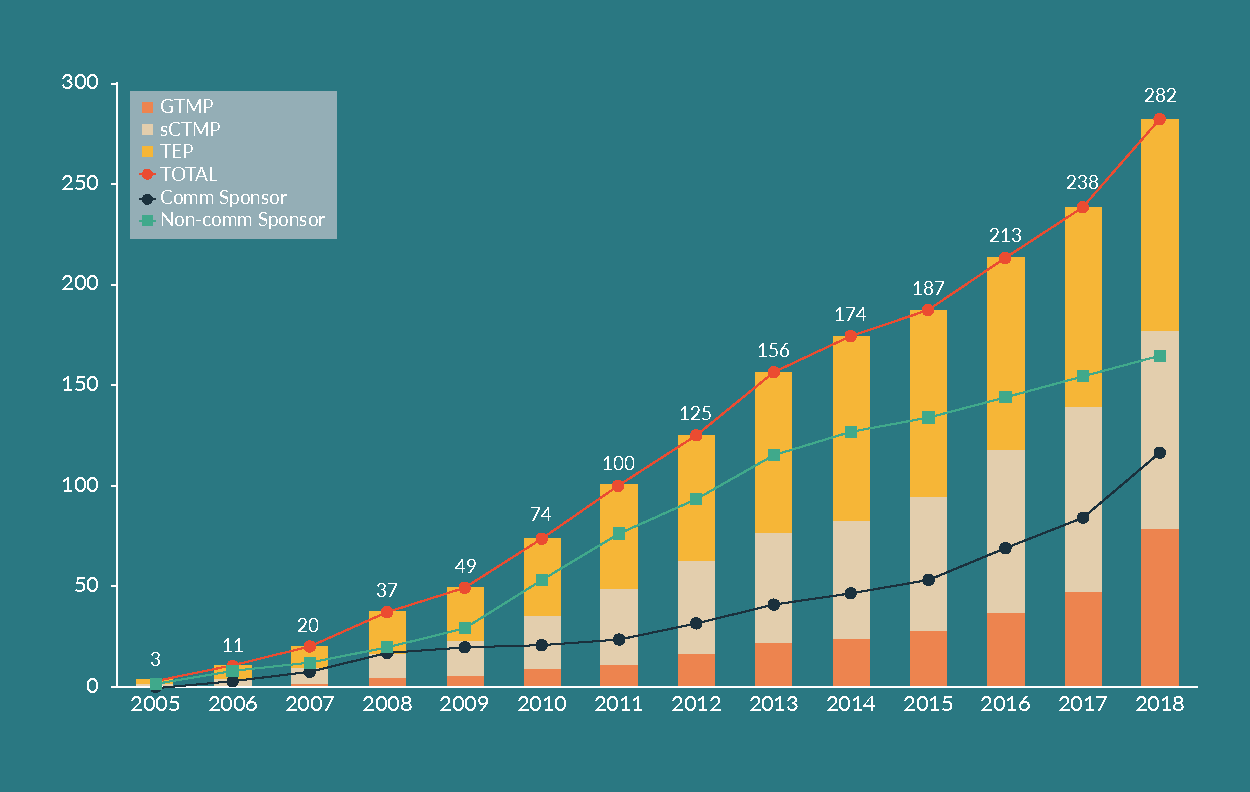
Clinical trials of advanced therapy investigational medicinal products in Spain: preparing for the European clinical trials regulation
J Estevez Alamo, M Timón, C González Gómez-Platero et al
08 November 2019
Regulatory Insight
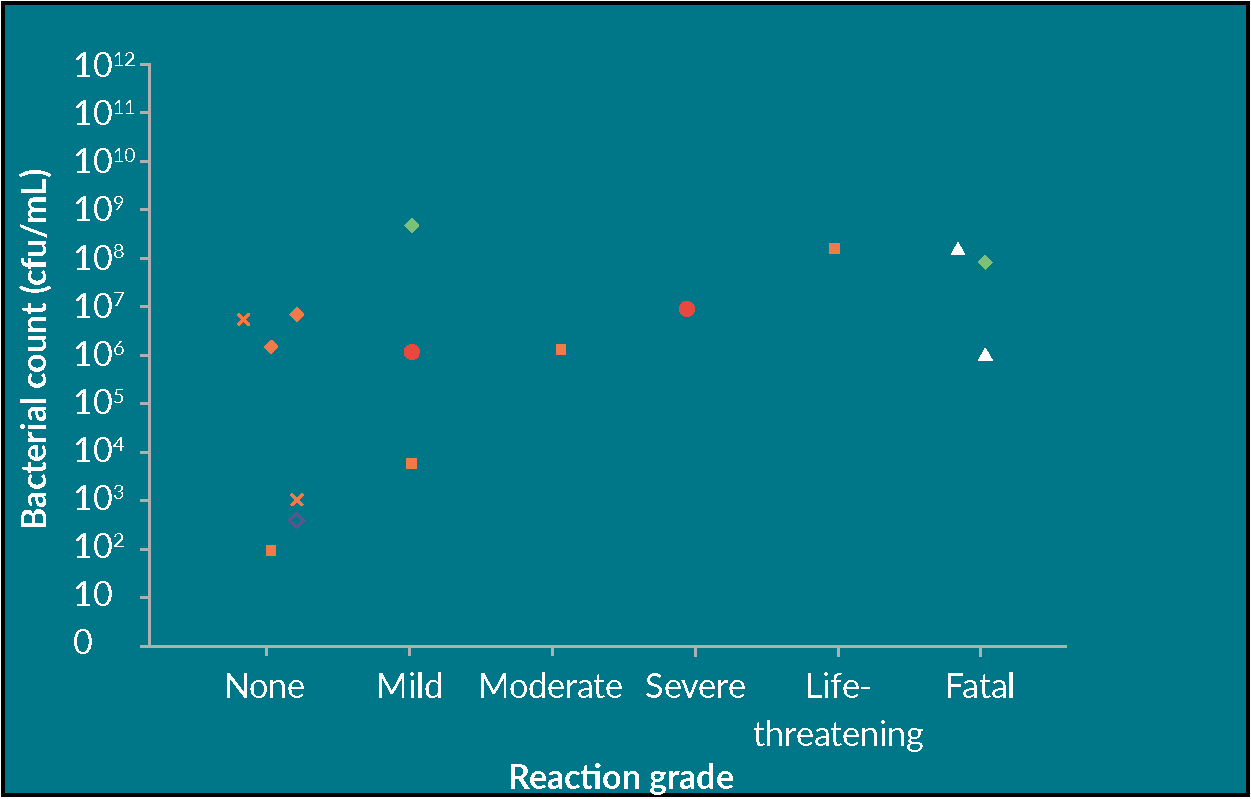
Sterility sampling of cell and gene therapy products
Timothy Wood
16 October 2019
Regulatory Insight

Preclinical considerations for assessment of cellular & gene therapy products: FDA perspective
Gaya Hettiarachchi, Wei Liang
22 July 2019
Regulatory Insight
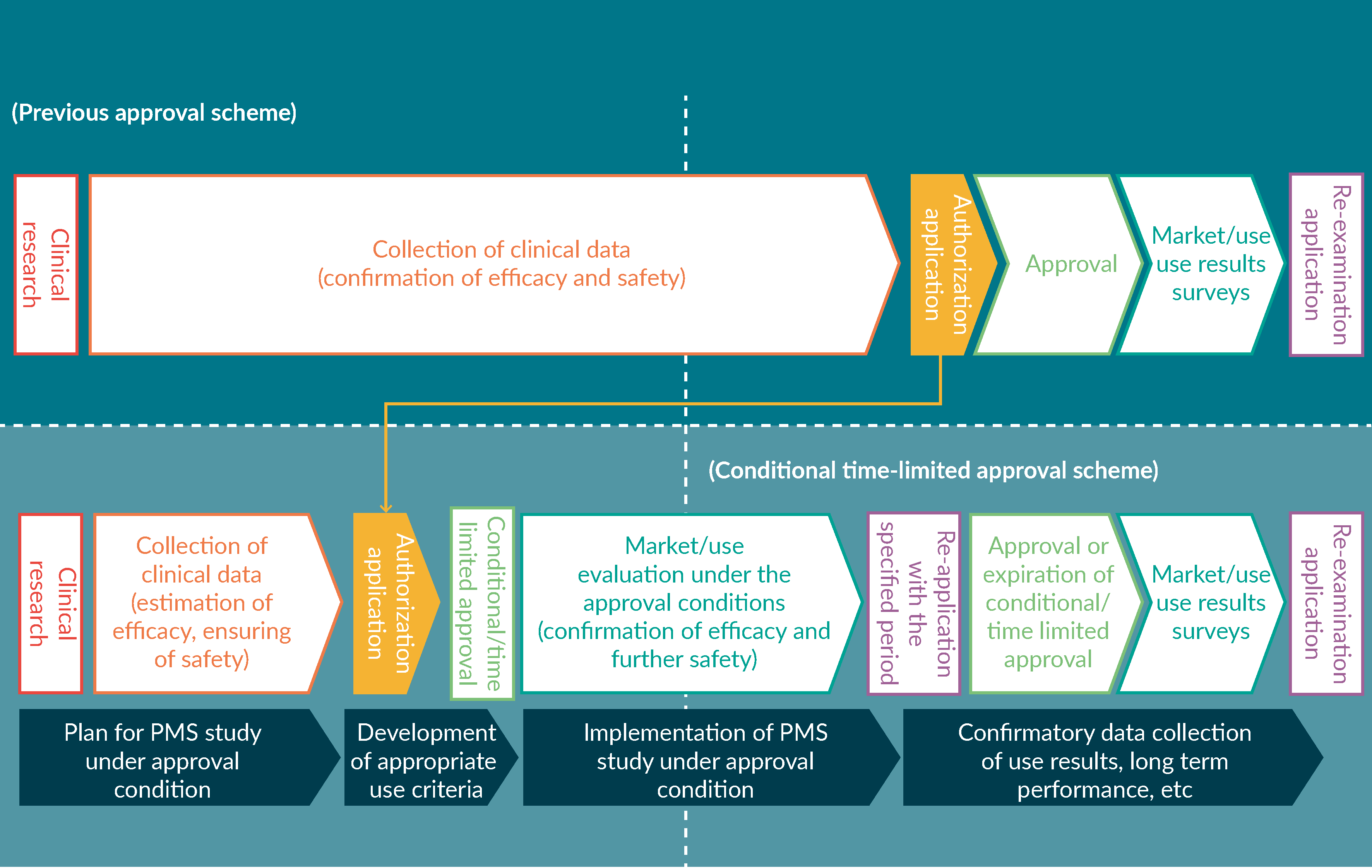
Experiences from Japan: conditional and time-limited approval – an early approval scheme for regenerative medical products
Y Maruyama, M Kasai, Y Fujiwara et al
24 June 2019
Regulatory Insight
Japan’s regulatory gamble and what it means for the Industry
Lee Buckler, Colin Novick
15 September 2015
Regulatory Insight
Regulatory viewpoints on the development of advanced stem cell–based medicinal products in light of the first EU-approved stem cell product
E Flory, P Gasparini, P Celis et al
15 September 2015
Regulatory Insight
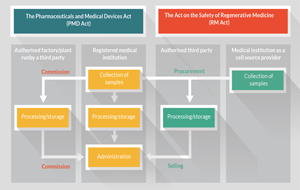
Japan’s regulatory framework: seeking to provide impetus to the commercialization of regenerative medicine products
Shintaro Sengoku, Mitsuya Sakurai, Yoshimi Yashiro
15 September 2015
Regulatory Insight