Filter by Interests
Filter by ContentType

Investing in early-stage stem cell therapy developers
Stijn Heessen, Kristian Tryggvason
21 February 2025
Viewpoint
Gene delivery: persistent challenges call for a reference measure
Max Ryadnov
22 October 2024
Viewpoint

Regulatory strategies for developing and manufacturing RNA-LNPs
Alexander Aust
05 April 2024
Viewpoint
Following the (mAb) leader: leveraging monoclonal antibody cell line development & banking CMC strategies for iPSC-derived cell therapies
Damien Fink
17 January 2024
Viewpoint

The current viral vector characterization landscape: from navigating evolving regulatory guidance to leveraging analytical tools for process development
Ning Ding
08 December 2023
Viewpoint
Designing cell therapy analytics for future success
Raymond Luke
21 September 2023
Viewpoint

Preclinical safety assessment in therapeutic genome editing
Roberto Nitsch
10 March 2023
Viewpoint

Addressing gene therapy translational R&D bottlenecks in the CNS
Anindya Sen
09 March 2023
Viewpoint
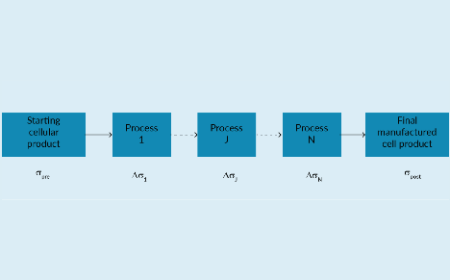
Producing cell-based therapeutic products with lot-to-lot consistency from highly variable starting cell products
William E Janssen, Scott R Burger
02 November 2022
Viewpoint

Analytical development for rapid response vaccines: start early, think ahead
Anna Särnefält, Ingrid Kromann
20 May 2022
Viewpoint

Considerations for demonstrating comparability of AAV processes
Laura Giersch
26 April 2022
Viewpoint

Process analytical technology tools for process monitoring in CGT product manufacturing
Wai Lam W Ling, Arun C Patel
18 January 2022
Viewpoint
Recent evolution in Process Analytical Technology for viral vectors
Tony Bou Kheir
20 August 2021
Viewpoint