Filter by Interests
Filter by ContentType
Efficient non-viral engineering of immune cells for cell therapy using circular single-stranded DNA
Hao Wu
13 January 2025
Viewpoint
Gene delivery: persistent challenges call for a reference measure
Max Ryadnov
22 October 2024
Viewpoint

Pioneering the future of non-viral genome engineering
Hao Wu
18 September 2024
Viewpoint

Expanding the therapeutic index of lipid nanoparticles: potential key for clinical translation success
Zhenghong Gao
12 June 2024
Viewpoint
Best practices and considerations for outsourcing with CDMOs
Joseph W Graskemper
11 April 2024
Viewpoint

The current viral vector characterization landscape: from navigating evolving regulatory guidance to leveraging analytical tools for process development
Ning Ding
08 December 2023
Viewpoint

Scale-up and automation of iPSC-derived cell therapy manufacturing
Dhruv Sareen, Jonathan Rodriguez, Hojae Lee
05 October 2023
Viewpoint
Harnessing the untapped potential of commensal viruses in genetic medicine
Joseph Cabral
04 May 2023
Viewpoint
Standardizing success: paving the way for streamlined viral vector manufacturing in gene therapy
Raquel Martín-Ibáñez
26 April 2023
Viewpoint
Setting up a one-stop shop: bringing viral vector manufacturing in-house
Danielle Steele
17 April 2023
Viewpoint
Transfection optimization for AAV production
Peter Boyce
03 April 2023
Viewpoint

The present hurdles and opportunities in our knowledge of AAV basic biology
Bijay Dhungel, John Rasko
17 May 2022
Viewpoint
Overcoming the immune-related shortfalls of AAV
Genine Winslow
10 May 2022
Viewpoint

Future trends in viral vector process & product development
Ramji Krishnan
28 April 2022
Viewpoint

Considerations for demonstrating comparability of AAV processes
Laura Giersch
26 April 2022
Viewpoint
Titration for research grade rAAV: understanding challenges and minimum requirements
Javier F Alcudia
06 December 2021
Viewpoint
From start-up to scale-up: a journey through AAV process development
Kevin Mouillesseaux
05 October 2021
Viewpoint
Viral vector gene delivery: balancing safety, efficacy and dose
Thijs Gerritzen
01 October 2021
Viewpoint

Overview of major AAV production platforms
Jote Bulcha
24 September 2021
Viewpoint
Recent evolution in Process Analytical Technology for viral vectors
Tony Bou Kheir
20 August 2021
Viewpoint
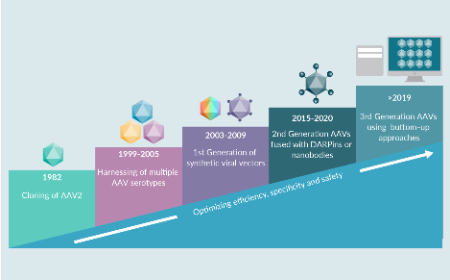
The dose makes the poison: next generation AAV vectors can save the day
Julia Fakhiri, Jihad El Andari
13 July 2021
Viewpoint