Filter by Interests
Filter by ContentType
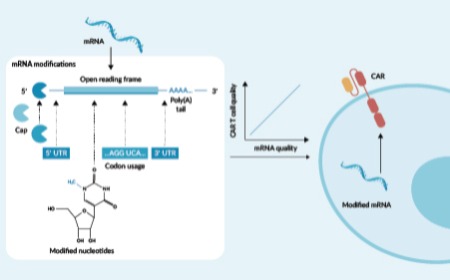
Advancing mRNA-based cell therapies: the crucial role of mRNA optimization for therapeutic efficacy
Christian Bär, Ulrich Blache, Sandy Tretbar
23 January 2025
Viewpoint

Bio-printing in the context of end-to-end scalable tissue-engineered ATMP manufacturing
Isaak Decoene, Ioannis Papantoniou
12 December 2024
Viewpoint

Industrializing cell therapy: how to bring curative therapies to all patients who need them?
Jens Vogel
20 November 2024
Viewpoint

Decentralizing gene and cell therapy manufacturing: changing tides or uphill battle
Magdi Elsallab
04 October 2024
Viewpoint
A pathway forward for CAR-NK cell therapy
Scott McComb
06 August 2024
Viewpoint
Enabling affordable access to CAR-T cell and other cellular gene therapy products
R Orentas, Y Xiong, I Oparaocha et al
13 May 2024
Viewpoint
Autologous induced pluripotent stem cell (iPSC)-derived therapies: a realistic solution for advancing regenerative medicine
Jarett Anderson, Timothy Nelson
17 January 2024
Viewpoint
Following the (mAb) leader: leveraging monoclonal antibody cell line development & banking CMC strategies for iPSC-derived cell therapies
Damien Fink
17 January 2024
Viewpoint

Scale-up and automation of iPSC-derived cell therapy manufacturing
Dhruv Sareen, Jonathan Rodriguez, Hojae Lee
05 October 2023
Viewpoint
Standardizing success: paving the way for streamlined viral vector manufacturing in gene therapy
Raquel Martín-Ibáñez
26 April 2023
Viewpoint
Setting up a one-stop shop: bringing viral vector manufacturing in-house
Danielle Steele
17 April 2023
Viewpoint
Transfection optimization for AAV production
Peter Boyce
03 April 2023
Viewpoint
Distributed manufacturing models for ATMPs: can they work: do we understand the pitfalls?
Mark Lowdell
02 March 2023
Viewpoint

Current trends in advanced therapy development & commercialization for rare diseases
Alan Boyd
28 February 2023
Viewpoint
Key considerations for early investment in the process development of cell-based therapeutics
Kelly Kemp, Sebastian Rieck
23 November 2022
Viewpoint

Building resilience into post-pandemic cell & gene therapy supply chains
Tiffany Clement, Cheryl Cox
18 November 2022
Viewpoint

The living cell supply chain: a product-specific, rational approach to management of cellular starting material donors & donations
William E Janssen, Scott R. Burger
18 November 2022
Viewpoint
Producing cell-based therapeutic products with lot-to-lot consistency from highly variable starting cell products
William E Janssen, Scott R Burger
02 November 2022
Viewpoint

Future trends in viral vector process & product development
Ramji Krishnan
28 April 2022
Viewpoint

Pandemic-related supply chain disruptions in cell therapy require rapid qualification for single-sourced materials
John Duguid, Paul Friedman
07 March 2022
Viewpoint

Process analytical technology tools for process monitoring in CGT product manufacturing
Wai Lam W Ling, Arun C Patel
18 January 2022
Viewpoint