
The comprehensive characterization of critical quality attributes (CQAs) for the safety and efficacy of cell and gene therapies remains a key challenge for the sector. Analytical development teams are required to address issues such as identification, characterization, and quantification of undesired contaminants such as mycoplasma.
With the CMC-related requirements of the US FDA, EMA, and other major regulatory agencies still evolving and steadily increasing in stringency, it is more important than ever to harness the cutting edge in analytical tools to improve the identification and measurement of these CQAs.
Fortunately, a new generation of analytical tools designed to meet the specific needs of cell therapy and viral vector production is arriving, offering the enhanced speed, sensitivity, and robustness that industry requires.
The following curated collection of content offers technical and strategic insights and practical advice relating to:
 Analytical development
Analytical development Cell therapy manufacturing
Cell therapy manufacturing Expression systems
Expression systems Mycoplasma testing
Mycoplasma testing Regulatory CMC
Regulatory CMC Residual DNA testing
Residual DNA testing Vector production
Vector production
Any Questions?
Content

Balancing precision and efficiency in cell therapy assays: low volume sampling for mycoplasma detection

Preparing for success in gene therapy analytical development
Preparing for success in gene therapy analytical development

Early-stage analytical development strategies for cell therapy
4 key lessons learnt from choosing in-house assay development
Leveraging dPCR for residual DNA and viral titer quantitation in advanced therapy manufacture
Analytical strategies for sterility and mycoplasma testing in biotherapies: from early development to production scale-up

5 dos and don'ts for regulatory compliance in AAV manufacturing
Overcoming challenges in cell therapy production: rapid sterility testing

What goes into developing an in-house method for quantitation of residual host cell DNA?

Regulatory considerations and validation strategies for mycoplasma testing for cell-based therapies

Addressing regulatory guidance for HEK293 cells and AAV-based therapeutics manufacturing
Simplifying residual DNA quantitation and biotherapeutic manufacturing

Streamlining nucleic acid extraction for biotherapy manufacturing: manual and automated solutions for success

Considerations for affinity capture in an AAV platform downstream process
Simplifying residual DNA quantitation in biotherapeutic manufacturing

Analyzing lentivirus particles using dPCR techniques

Considerations for affinity capture in an AAV platform downstream process

Improving biopharmaceutical quality and safety by implementing a microbial identification strategy in the workflow
Regulatory considerations & validation strategies for mycoplasma testing for cell-based therapies

Navigating evolving regulatory CMC guidance in the AAV gene therapy field

Addressing regulatory guidance for HEK293 cells & AAV-based therapeutics manufacturing
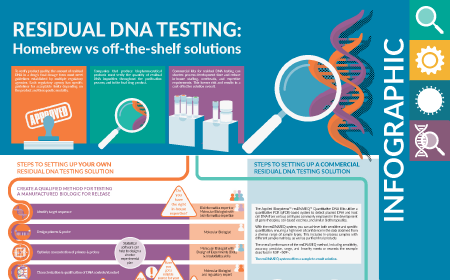
Residual DNA testing: Homebrew vs off-the-shelf solutions: INFOGRAPHIC
Regulatory considerations and validation strategies for mycoplasma testing for cell-based therapies
Addressing regulatory guidance for HEK293 cells and AAV-based therapeutic manufacturing
Environmental monitoring: optimizing microbial control in cell & gene therapy workflows
Optimizing the cell therapy patient journey through integrated CRO/CDMO partnership

Navigating evolving regulatory CMC guidance in the AAV gene therapy field

Simple analytical tools for detection of impurities in biologics produced using the Sf-baculovirus platform
Environmental monitoring: optimizing microbial control in cell and gene therapy workflows

Navigating evolving regulatory CMC guidance in the AAV gene therapy field

Analytical innovation: meeting the demands of commercial viral vector manufacture

Lentiviral titer determination: rapid & robust molecular methods suitable for validation
Lentiviral titer determination: rapid & robust molecular methods suitable for validation

Mycoplasma testing: regulatory guidance and strategies for cGMP cell and gene therapy manufacturing

Robust quantitation of residual host cell and plasmid DNA & oncogenic fragments in HEK-based viral vector manufacturing
Simplifying analytical development of viral vector production: robust and sensitive methods for common expression systems

Removing technological barriers to efficient large-scale LV vector production

Removing technological barriers to efficient large-scale LV vector production
Simplifying analytical development for viral vector production: robust and sensitive methods for common expression systems
Key factors to consider for successful cell therapy manufacturing: a case study
Residual DNA testing in viral vector manufacture: exploring the challenges and solutions

Expression systems for viral vector production: advantages of the Sf9 baculovirus system and simple solutions to address its specific analytical challenges
Manufacturing and analytics for lentivirus and AAV vectors: a visual and audio guide

Development & validation of a robust commercial solution for measuring residual kanamycin-resistant plasmid DNA
Expression systems for viral vector production: advantages of the Sf9 Baculovirus system and simple solutions to address its specific analytical challenges

Mycoplasma detection in cell therapy products: GMP-compliant implementation & validation of a commercial real-time PCR assay for routine quality control & lot release

Viral vector production process intensification: analytics, automation, in-line testing and more

Viral vector production process intensification: analytics, automation, in-line testing, and more
Scalable AAV manufacturing – Addressing challenges across the workflow

Combining state-of-the-art production, purification and analytics to optimize AAV manufacturing for clinical and commercial gene therapies

AAV vector process development: achieving high purity and high yield – experiences from the frontline
