March 2020 Issue
Volume 6 Issue 2

Featuring
Issue Spotlight:
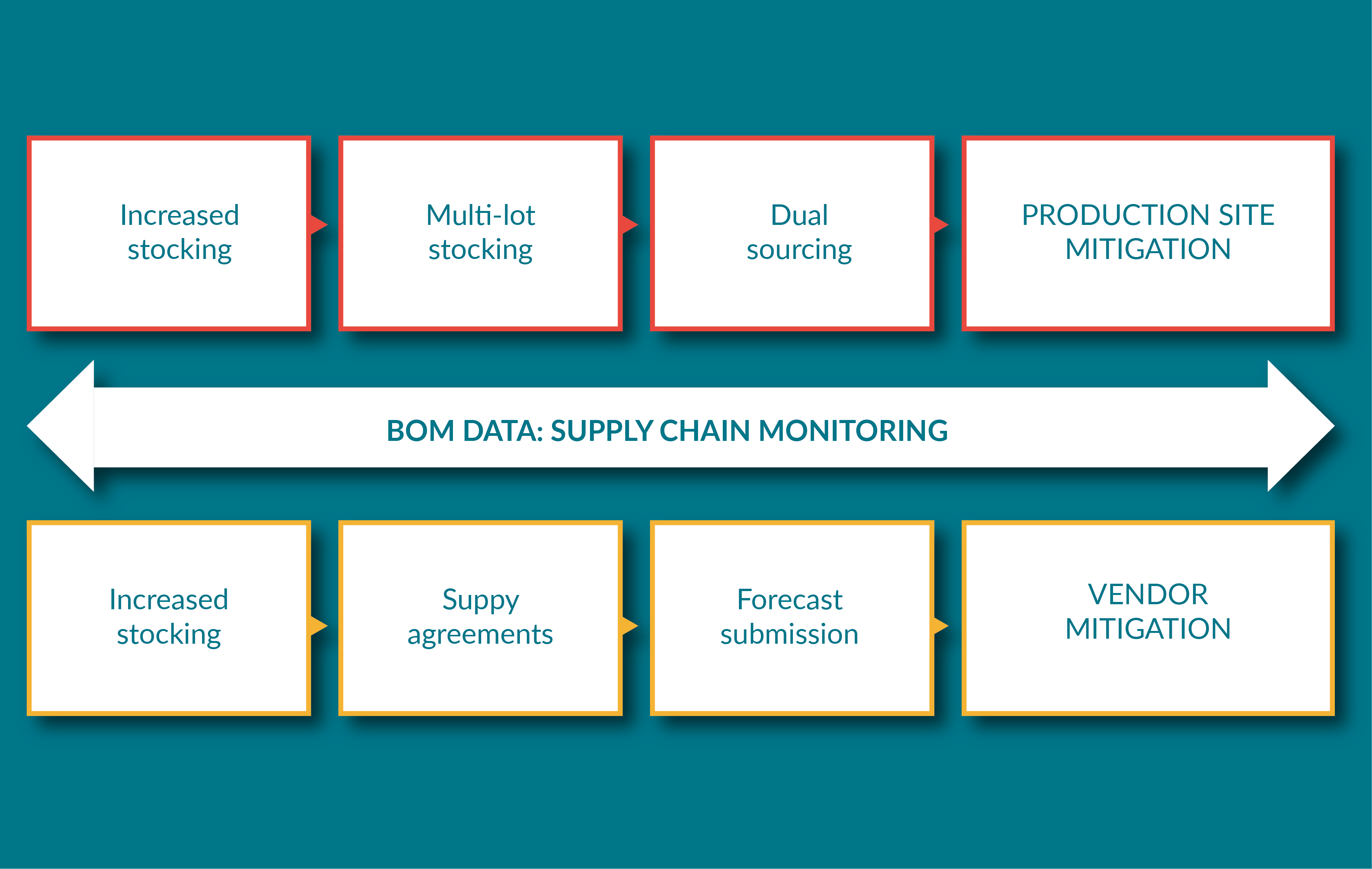
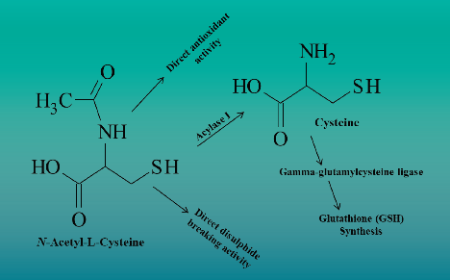
Raw and starting materials: troubleshooting supply, management and optimisation issues
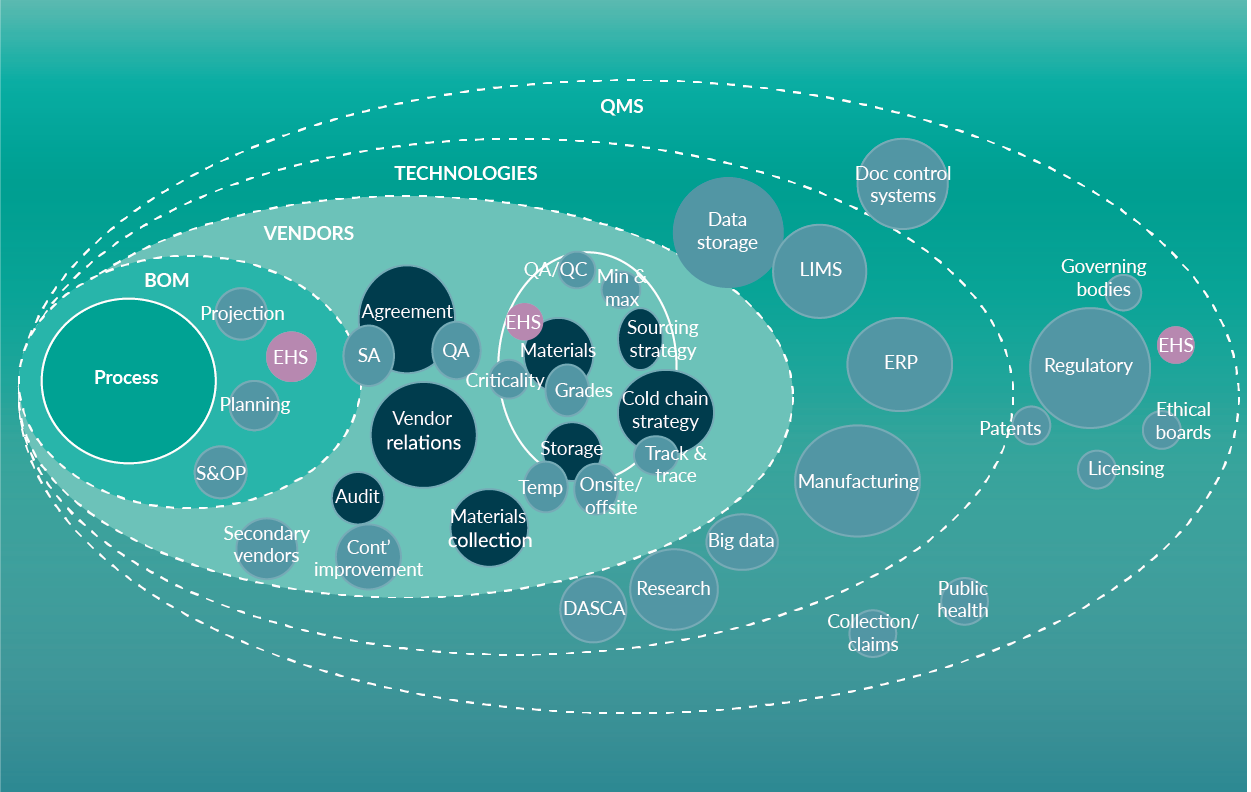
Supply Chain focus: Global cell and gene therapy supply chain strategies at commercial scale 2020
Other articles
Webinars