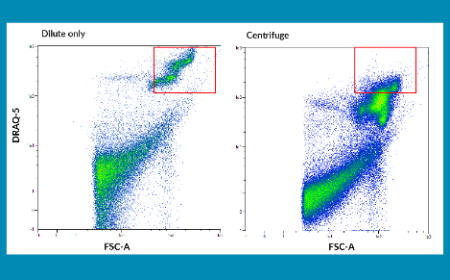
February 2021 Issue
Volume 7 Issue 1

Featuring
Issue Spotlight:
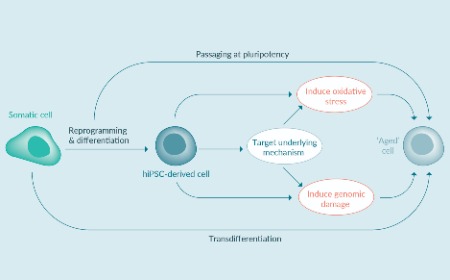
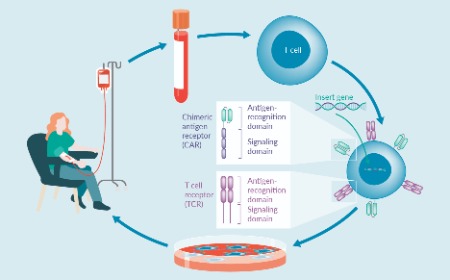
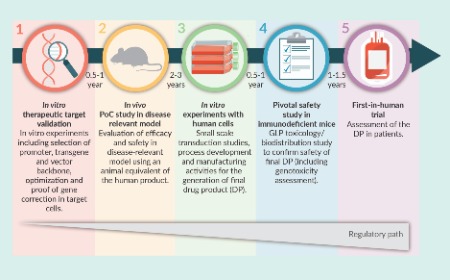
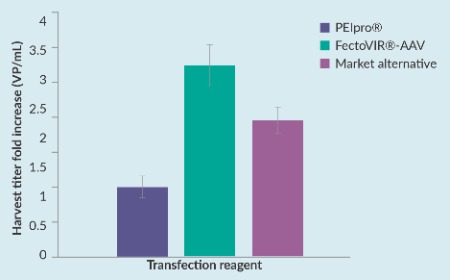
Preclinical/translational tools and strategies
Other articles
Webinars