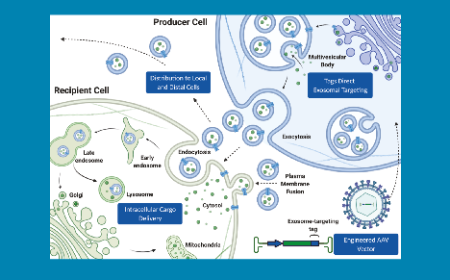
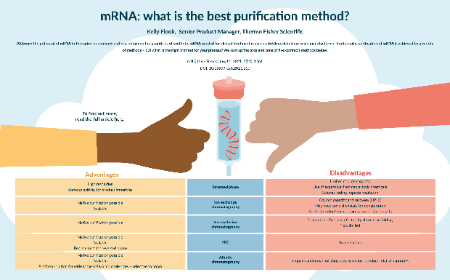
November 2021 Issue
Volume 7 Issue 11

Featuring
Issue Spotlight:
Cell therapy bioprocessing and automation
Vectors focus: Downstream Bioprocessing 2021
Other articles
Webinars