CAR T cell therapies in pediatric oncology: access and future development by distributed academic cell manufacturing
Cell & Gene Therapy Insights 2019; 5(5), 9-19
10.18609/cgti.2019.002
CAR T cell therapy is a major innovation in the treatment of B-cell malignancies. Prospective clinical evaluation within large, risk-stratified international multi-center studies is needed to establish its value beyond the salvage of refractory disease. To perform these trials, the academic community needs access to safe and effective uniform CAR T cell products across international borders. Novel automation technologies enable decentralized manufacturing of highly standardized CAR T cell products in academic GMP facilities experienced with the production of patient-individual cell therapies. Academic cell manufacturing in addition to products from pharmaceutical companies will allow the informed dissemination of CAR T cell therapy to patients who benefit from this modality. It will feed back into further preclinical improvements, enable clinical researchers to define the optimum position of CAR T cell therapy within multimodal therapies, and help advance this approach to treat other cancers in both children and adults.
Submitted for peer review: Nov 11 2018 Published: Jul 4 2019
The development of effective therapies for childhood leukemia is a major success story in medicine. The key to long-term remission and cure was the introduction of combination chemotherapies and their systematic improvement in randomized clinical trials [1–4]. Risk-stratified large national and international multi-center trials today are the standard of care in pediatric hematology and oncology [5]. A firsT cell therapy was introduced into leukemia therapy in the 1960s in the form of allogeneic hematopoietic stem cell transplantation (HSCT) [6,7]. The value of HSCT in pediatric patients has been systematically evaluated in both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) in academic multicenter trials, and was found to add substantial benefit for high-risk populations of patients [8,9]. Despite these advances, a significant proportion of patients with acute leukemia fail current therapies including HSCT, and relapse after transplant remains largely incurable [10]. Moreover, HSCT still has a high risk of treatment-related mortality and long-term morbidities, caused by organ toxicities from the conditioning regimen and by acute and chronic allogeneic immune responses. Together, these limit both the duration and the quality of life in many survivors. Additional modalities are clearly needed to further improve leukemia therapy.
Targeted immunotherapies or immunotoxins directed against B lineage antigens have shown high potential to eliminate chemorefractory disease. The first-in-class bispecific T-cell engager blinatumomab binds CD19 on leukemic cells and CD3 on T cells, leading to T-cell activation and target cytolysis [11]. Blinatumomab as a single agent is potent to induce remissions in patients with refractory CD19-positive leukemias. In a pediatric Phase 2 study, 39% (27 of 70) of patients treated with blinatumomab at the recommended dose achieved complete remissions, of which 52% had an MRD-negative response [12]. Blinatumomab has been approved for the treatment of relapsed and refractory precursor B-cell ALL in adults and more recently in children [12,13].
Another off-the-shelf agent directed at a B lineage antigen is the CD22-specific immunotoxin inotuzumab-ozagamicin. Engagement of CD22 on the cell surface leads to internalization and release of the toxic component, calicheamicin, which induces cell death by breaking double-stranded DNA [14]. Based on a randomized study demonstrating a significantly higher rate of complete remissions in patients with refractory ALL treated with this agent compared to standard therapy, the agent was approved for adult ALL [15]. Inotuzumab-ozagamicin is still undergoing early-phase clinical investigation in pediatric patients.
Patient-individual cellular therapy with CD19-specific chimeric antigen-receptor (CAR) gene-modified T cells has emerged as an additional treatment modality for patients with refractory B-cell malignancies, with impressive clinical benefit in both ALL and non-Hodgkin lymphomas. The principle of CAR T cell therapy was established by academic researchers, starting with Zelig Eshhar´s observation in the late 1980s that CARs can activate T cells [16]. CARs consist of the antigen-binding domain of a monoclonal antibody linked to costimulatory and T-cell receptor-derived signaling domains. CAR genes are introduced into T cells by viral gene transfer ex vivo, followed by adoptive transfer to the patient (Figure 1).
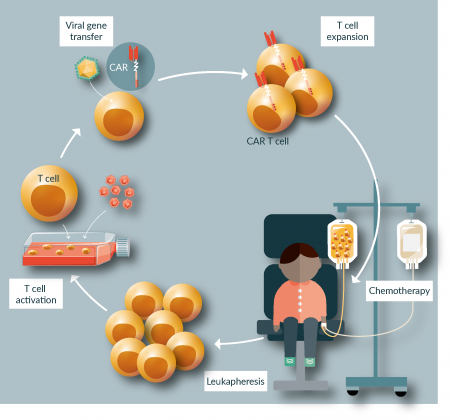
Figure 1: CAR T cell therapy. Treatment with CAR T cells involves manufacturing of an apheresis product from the patient, manufacturing of a CAR T cell product, preconditioning chemotherapy and administration of CAR T cells, usually as a single infusion.
Upon engagement of the target antigen on the cell surface, CAR gene-modified T cells receive potent activation stimuli leading to target cytolysis, cytokine release and clonal proliferation [17]. The majority of CAR T cell therapeutics developed to date target the B lineage antigen CD19. Lymphodepleting chemotherapy followed by administration of autologous CD19-specific CAR T cells can induce complete remissions in situations where cytotoxic agents, HSCT and even CD19-targeted immunotherapy with blinatumomab have failed [12,13,18–23]. Some CAR T cell products can generate long-term remissions lasting for more than 12 months in a significant proportion of patients [19]. Even though follow-up data is still limited, there is reason to believe that CAR T cell therapy can cure at least some patients with previously incurable leukemia or lymphoma.
Acute off-target toxicities of CD19 CAR T cell therapy include cytokine release syndrome (CRS) and neurotoxicity. Secretion of high levels of inflammatory cytokines in response to T-cell activation by CARs causes endothelial activation, leading to hemodynamic instability, capillary leak and coagulopathy [24]. CRS can be successfully managed with the IL-6 receptor antagonist tocilizumab [23]. Neurotoxicity becomes manifest as an encephalopathy-like syndrome with headaches, hallucinations, seizures, and behavioral abnormalities. It is often associated with CRS and is explained by a breakdown of the blood–brain barrier by enhanced endothelial permeability and entry of high levels of cytokines into the central nervous system [25]. Rare fatal toxicities have been reported, all in adult patients and associated with high CAR T cell doses and/or high disease burden [18,26–28]. An expected consequence of the on-target/off-tumor toxicity of CD19-specific CAR T cells against normal B cells and their CD19-expressing precursors is hypogammaglobulinemia by B-cell aplasia. It persists as long as functional CAR T cells are detectable in the blood [20,23] and can be effectively managed by regular parenteral substitution of immunoglobulins.
Treatment benefit of two CD19-specific CAR T cell products compared to a historical controls has led to marketing authorization both in North America and in Europe, including a pediatric indication. Tisagenlecleucel was approved for the treatment of patients with recurrent and/or refractory ALL up to the age of 25 years and adult patients with recurrent and/or refractory diffuse large B-cell lymphoma (DLBCL), and axicabtagene ciloleucel for the use in adult patients with recurrent and/or refractory DLBCL or primary mediastinal B-cell lymphoma.
The academic community is now confronted with the task of establishing the value of this promising new treatment modality by introducing CAR T cell therapy into the current complex treatment algorithms. Now that CAR T cell immunotherapy has been approved for patients with refractory disease, we must explore its potential for adding efficacy to or even replacing other therapies such as HSCT and cytotoxic drug treatment. Indeed, if CAR T cell therapy is safe and successful in high-risk leukemia, then we will need to evaluate CAR T cell therapy even as frontline treatment and for standard-risk disease. Due to the high complexity and cost of CAR T cell therapy as well as the acute toxicities and potential late effects, for example sustained B-cell depletion and hypogammaglobulinemia when CD19 is used as target, superiority will have to be clearly demonstrated. Pediatric B-cell cancers are rare diseases [29], and their state-of-the art treatment is complex. Thus, the optimal role of CAR T cell therapy can only be assessed in multi-center trials performed in national and international consortia that allow randomization of large numbers of patients. An essential prerequisite for such studies is to make the new agent available to patients and academic investigators. Securing reliable and affordable access to CAR T cell products that fulfill the highest quality standards is a critical next step for advancing treatment of B-cell cancers.
A first example of a cell therapy effectively introduced into multimodal treatment algorithms in childhood leukemias is HSCT. Advancing cell therapy by HSCT from its introduction in the 1960s [6,7] to the therapeutic standard it has become today has taken many years of refinement and optimization. Progress has been driven by the concerted action of the academic hematology and transfusion medicine community and led to significant reduction of transplant-associated lethality in both pediatric and adult patients over the past 20 years [30,31]. Harmonization of transplantation procedures in pediatric leukemia patients was achieved by performing HSCT within large prospective international multi-center trials [31], which have now started addressing randomized research questions [32]. Although transplants and even advanced HSCT products such as grafts depleted of selective cell populations are far less complex cell products than CAR T cells, experience from HSCT is transferrable in several aspects. One of the greatest challenges in extending the paradigm of multi-center clinical trials to HSCT has been the variability of the biological product. Hematopoietic stem cell products are living therapeutics varying in donor identity, origin from blood or bone marrow, and cell composition. While CAR T cell manufacturing adds a new dimension of cell engineering since it requires introduction of a gene for safe and reliable expression, interindividual variability is a shared feature between the two types of cell products. Moreover, both products must be manufactured separately for each individual patient according to good manufacturing practice (GMP) in authorized manufacturing centers to comply with pharmaceutical quality standards and to ensure adequate levels of safety for human use. For transplants, these standards have been successfully established in the academic setting to the degree that beyond unmanipulated hematopoietic stem cell grafts, academic centers now produce more and more complex individualized cell products. Positive selection of CD34+ hematopoietic stem cells or depletion of selective immune cell populations, such as CD3+ T cells or T-cell receptor /+ T cells and CD19+ B cells, allows HLA barriers to be overcome for transplantation even from HLA haploidentical donors [33–35]. Other types of cells, such as mesenchymal stem cells, are now being evaluated in clinical trials to prevent and treat graft-versus-host disease (GVHD) [36]. Moreover, T-cell products are manufactured from stem cell donors to treat impending relapse with unmodified donor lymphocyte infusions, or viral complications in patients post HSCT by ex vivo antigen selection of virus-specific T cells [37,38].
In contrast to HSCT and antiviral adoptive T-cell therapy, in which the products and the procedures are both institution based, access to CAR T cells currently follows a pharmaceutical model (Figure 2A). The hospital obtains a patient-individual leukapheresis product and sends it to a company, which uses this product to manufacture the gene-engineered CAR T cell product and return it to the hospital to be administered to the patient. This model has several flaws. Disease control until the CAR T cell product is available requires antiproliferative chemotherapy, so-called bridging therapy, which may by unsuccessful in aggressive disease refractory to cytotoxic agents, resulting in rapid progression and death or adverse events precluding infusion. Protocols used in previous studies have limited the use of alternative antileukemic agents to be used in this situation, for example have excluded treatment with CD19-targeted agents such as blinatumomab [19] for concerns that pretreatment with CD19-targeted agents could favor antigen-negative escape. As a consequence, manufacturing failures, which occur in a significant proportion of patients [19], may prevent the patient from receiving a treatment capable of extending survival and enhancing the quality of life even if ultimately non-curative. Examples of long-term responders to CD19-specific CAR T cell therapy post blinatumomab now argue against a significant negative effect of the T-cell engager on subsequent CAR T cells targeting the same antigen [21,23], and ongoing treatment protocols have started allowing B-cell targeted agents for more effective bridging therapy to CAR T cells.
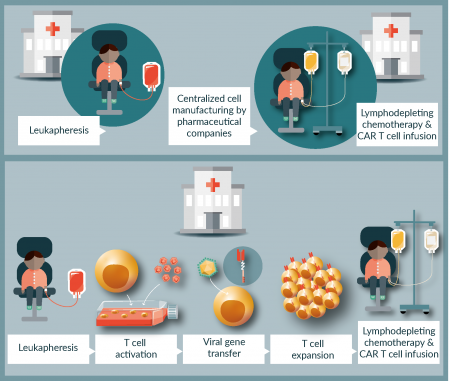
Figure 2: Two models of CAR T cell manufacturing for advanced-phase clinical trials. (A) Pharmaceutical cell production. Following T-cell apheresis at the local hospital, the product is sent to the pharmaceutical company for centralized CAR T cell manufacturing, then transferred to the hospital for performing preconditioning chemotherapy and CAR T cell administration. (B) Decentralized academic cell production: CAR T cell products are generated by in-house manufacturing in accredited academic facilities at the local hospital according to an established standard procedure, then transferred to the patient following lymphodepletive preconditioning therapy.
Times between apheresis and administration of a product manufactured outside the hospital can be extended beyond manufacturing by long shipping distances and international borders complicating delivery. The manufacturing process often lacks transparency, which is needed for the local management of the patient and to respond to the questions and needs of the families during the time in which the product is being manufactured. Importantly, studies to establish the value of industrially manufactured CAR T cells used in academic multi-center trials will require close cooperation between companies and academic investigators, and logistic solutions that allow distribution of centrally prepared CAR T cell products across international borders. Last, but by no means least, the high cost of the currently approved pharmaceutical products together with the cost of prior treatment and of toxicity management will profoundly limit broad application and systematic evaluation of the ultimate place of CAR T cell therapy in cancer therapy.
A way out of these limitations is to enable and expand additional decentralized in-house manufacturing and distribution of CAR T cells by experienced academic facilities who can comply with established and uniform GMP standard operation procedures (Figure 2B). Compared to bone marrow and peripheral-blood derived stem cell products and even antigen-selected virus-specific T cells, CAR T cell manufacturing adds several additional levels of complexity [39]. Most importantly, safe and effective gene transfer is an essential part of the process. T cells must be isolated from the peripheral blood leukapheresis product, activated, genetically engineered to express the CAR construct, expanded and formulated into the final product. Standard GMP manufacturing of CAR T cells involves multiple manipulation steps requiring highly skilled and experienced staff in a cleanroom environment. To minimize the risks, labor intensity and cost and to improve the robustness and scalability of CAR T cell manufacturing, technological advances need to be developed that automate and simplify the procedure [40], allowing decentralized manufacturers to produce large numbers of highly equivalent products at affordable prices.
Because of their inherent biology, cell products generated from individual humans can never reach the level of inter-product comparability of classical drugs. Each autologous CAR T cell product derives from a highly diverse human T-cell repertoire, which has been shaped by a variable history of antigen exposures and, in leukemia patients, has further been affected by pretreatment with cytotoxic agents and immunosuppressive therapies. To reduce this heterogeneity and increase product harmonization it may be necessary to combine uniform manufacturing standards with plans to conduct apheresis for future CAR T cell therapy at defined timepoints in treatment protocols. Following assessment of the safety and efficacy of CAR T cell products manufactured according to standardized procedures at qualified individual sites in Phase 1/2 clinical trials, they can be used in academia-initiated advanced-Phase clinical multi-center trials. The trials will establish the value of CAR T cell therapy in comparison with alternative agents and with HSCT and define their optimal position in existing treatment algorithms. In addition to the central patient registration and analysis of all relevant clinical parameters that these studies provide, they must be accompanied by an immune monitoring program that maximizes the gain of information and allows the critical hurdles for the production of optimal cell products and their clinical use to be identified. Such programs are well established in the academic reference laboratory facilities integrated into current multi-center trials. Academic multi-center trials also are an optimal platform for establishing uniform regulation to assure the highest standards of quality in the care of the patients receiving CAR T cell therapy.
Beyond B-cell cancers, technologies for decentralized academic cell manufacturing will also facilitate for the academic investigators to advance the technology towards solid tumors where CAR T cell therapy so far has been less effective. Strategies are needed that enable T-cell trafficking into the solid tumor microenvironment, enhance local T-cell proliferation and persistence and overcome the protective tumor microenvironment of solid tumors. Moreover, adequate antigens must be identified and novel generations of CARs established that avoid both on-target toxicity to healthy cells and overcome heterogeneity of expression of targets on subpopulations of cancer cells. Approaches targeting single antigens may not be sufficient for durable long-term antitumor responses. Instead, novel T cell engineering strategies are needed to enhance potency and selectivity, for example CARs that recognize an expression pattern unique to tumor site rather than an individual antigen [41].
Successful research in this area will have to coordinately consider all aspects of cancer and immune biology. Even optimized CAR targeting strategies are highly unlikely to make an impact in solid cancers on their own. Cotargeting inhibitory pathways such as PD-1 [42] or engineering CAR T cells for locoregional production of interleukin (IL-)18 [43–45] may help to enhance the function of CAR T cells in solid tumors. Also in solid tumors, cell therapy will have to be embedded into multimodal treatment protocols to study the benefit for patient subgroups in large comparative clinical trials. These studies will continue to be performed by academic study groups of the scientific community within their established infrastructure [46].
While initial CAR T cell trials were academia-driven and industry has relied on academic research for acquiring pivotal knowledge in the manufacture of individualized cell-based gene therapy products it then had a significant role to develop CAR T cell technology to first approval. Even commercial manufacturers with large budgets and investment are facing challenges in the production of patient-individual CAR T cell products. To enable cell manufacturing in the academic setting, closed-system and automation technologies for cell manufacturing are needed, along with standard processes and components for distributed manufacture and quality control. Support from industry will substantially contribute to the success of investigator-initiated clinical trials evaluating cellular therapeutics. Alongside distributed academic small-scale manufacturing, commercial CAR T cell products manufactured at large central sites may be well suited for larger scale treatments in more common diseases or to supply access when their application broadens toward frontline care.
Finally, all stakeholders are aware of the fact that CAR T cells are only one manifestation of the developing area of cellular therapy, and others such as transgenic T-cell receptor modified cells will follow soon. Reminiscent of HSCT it will again need the concerted activity of the academic community in hematology, oncology and transfusion medicine together with industry and regulating authorities to bring this modality to full fruition.
Financial & Competing Interests Disclosure
The authors have no relevant financial involvement with an organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock options or ownership, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
References
1. George P, Hernandez K, Hustu O, Borella L, Holton C, Pinkel D. A study of “total therapy” of acute lymphocytic leukemia in children. J. Pediatr. 1968; 72: 399-408. CrossRef
2. Henze G, Langermann HJ, Lampert F, Neidhardt M, Riehm H. [ALL therapy study 1971-1974 of the German working group for leukemia research and therapy in childhood: prognostic significance of initial features and different therapeutic modalities (author’s transl)]. Klin. Padiatr. 1979; 191: 114–26.
3. Frei E 3rd, Holland JF, Schneiderman MA et al. A comparative study of two regimens of combination chemotherapy in acute leukemia. Blood 1958; 13: 1126–48. Website
4. Moricke A, Zimmermann M, Reiter A et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia 2010; 24: 265–84. CrossRef
5. Rossig C, Juergens H, Schrappe M et al. Effective childhood cancer treatment: the impact of large scale clinical trials in Germany and Austria. Pediatr Blood Cancer 2013; 60: 1574–81. CrossRef
6. Mathe G, Amiel JL, Schwarzenberg L et al. Successful Allogenic Bone Marrow Transplantation in Man: Chimerism, Induced Specific Tolerance and Possible Anti-Leukemic Effects. Blood 1965; 25: 179–96.
7. Buckner CD, Epstein RB, Rudolph RH, Clift RA, Storb R, Thomas ED. Allogeneic marrow engraftment following whole body irradiation in a patient with leukemia. Blood 1970; 35: 741–50.
8. Klusmann JH, Reinhardt D, Zimmermann M et al. The role of matched sibling donor allogeneic stem cell transplantation in pediatric high-risk acute myeloid leukemia: results from the AML-BFM 98 study. Haematologica 2012; 97: 21–9.
9. Eckert C, Henze G, Seeger K et al. Use of allogeneic hematopoietic stem-cell transplantation based on minimal residual disease response improves outcomes for children with relapsed acute lymphoblastic leukemia in the intermediate-risk group. J. Clin. Oncol. 2013; 31: 2736–42. CrossRef
10. Kuhlen M, Willasch AM, Dalle JH et al. Outcome of relapse after allogeneic HSCT in children with ALL enrolled in the ALL-SCT 2003/2007 trial. Br. J. Haematol. 2018; 180: 82–9. CrossRef
11. Bargou R, Leo E, Zugmaier G et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 2008; 321: 974–7. CrossRef
12. von Stackelberg A, Locatelli F, Zugmaier G et al. Phase I/Phase II Study of Blinatumomab in Pediatric Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2016; 34: 4381–9. CrossRef
13. Topp MS, Gokbuget N, Stein AS et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015; 16: 57–66. CrossRef
14. DiJoseph JF, Armellino DC, Boghaert ER et al. Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood 2004; 103: 1807–14. CrossRef
15. Kantarjian HM, DeAngelo DJ, Stelljes M et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2016; 375: 740–53. CrossRef
16. Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. USA 1993; 90: 720–4. CrossRef
17. Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat. Biotechnol. 2002; 20: 70–5. CrossRef
18. Turtle CJ, Hanafi LA, Berger C et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 2016; 126: 2123–38. CrossRef
19. Maude SL, Laetsch TW, Buechner J et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018; 378: 439–48. CrossRef
20. Lee DW, Kochenderfer JN, Stetler-Stevenson M et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015; 385: 517–28. CrossRef
21. Gardner RA, Finney O, Annesley C et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 2017; 129: 3322–31. CrossRef
22. Brentjens RJ, Davila ML, Riviere I et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013; 5: 177ra38. CrossRef
23. Maude SL, Frey N, Shaw PA et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014; 371: 1507–17. CrossRef
24.Hay KA, Hanafi LA, Li D et al. Kinetics and Biomarkers of Severe Cytokine Release Syndrome after CD19 Chimeric Antigen Receptor-modified T cell Therapy. Blood 2017; 130: 2295–306.CrossRef
25. Gust J, Hay KA, Hanafi LA et al. Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017; 7: 1404–19 CrossRef
26. Turtle CJ, Hay KA, Hanafi LA et al. Durable Molecular Remissions in Chronic Lymphocytic Leukemia Treated With CD19-Specific Chimeric Antigen Receptor-Modified T cells After Failure of Ibrutinib. J. Clin. Oncol. 2017; 35: 3010–20. CrossRef
27. Turtle CJ, Hanafi LA, Berger C et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci. Transl. Med. 2016; 8: 355ra116. CrossRef
28. Park JH, Riviere I, Gonen M et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018; 378: 449–59. CrossRef
29.Steliarova-Foucher E, Colombet M, Ries LAG, Moreno F, Dolya A, Bray F, Hesseling P, Shin HY, Stiller CA, contributors I-. International incidence of childhood cancer, 2001-10: a population-based registry study. Lancet Oncol. 2017; 18: 719–31. CrossRef
30. Gooley TA, Chien JW, Pergam SA et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N. Engl. J. Med. 2010; 363: 2091–101. CrossRef
31. Peters C, Schrappe M, von Stackelberg A et al. Stem-cell transplantation in children with acute lymphoblastic leukemia: A prospective international multicenter trial comparing sibling donors with matched unrelated donors – The ALL-SCT-BFM-2003 trial. J. Clin. Oncol. 2015; 33: 1265–74. CrossRef
32. Willasch AM, Peters C, Sedlacek P et al. Myeloablative Conditioning for First Allogeneic Hematopoietic Stem Cell Transplantation in Children with ALL: Total Body Irradiation or Chemotherapy? – a Multicenter EBMT-PDWP Study. Blood 2017; 130: 911. Website
33.Aversa F, Tabilio A, Velardi A et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N. Engl. J. Med. 1998; 339: 1186–93. CrossRef
34. Bertaina A, Merli P, Rutella S et al. HLA-haploidentical stem cell transplantation after removal of alphabeta+ T and B cells in children with nonmalignant disorders. Blood 2014; 124: 822–6. CrossRef
35. Lang P, Feuchtinger T, Teltschik HM et al. Improved immune recovery after transplantation of TCRalphabeta/CD19-depleted allografts from haploidentical donors in pediatric patients. Bone Marrow Transplant. 2015; 50 Suppl. 2: S6–10. CrossRef
36. Le Blanc K, Frassoni F, Ball L et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008; 371: 1579–86. CrossRef
37. Tzannou I, Papadopoulou A, Naik S et al. Off-the-Shelf Virus-Specific T cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation. J. Clin. Oncol. 2017; 35: 3547–57. CrossRef
38. Feucht J, Opherk K, Lang P et al. Adoptive T-cell therapy with hexon-specific Th1 cells as a treatment of refractory adenovirus infection after HSCT. Blood 2015; 125: 1986–94. CrossRef
39. Hartmann J, Schussler-Lenz M, Bondanza A, Buchholz CJ. Clinical development of CAR T cells-challenges and opportunities in translating innovative treatment concepts. EMBO Mol. Med. 2017; 9: 1183–97. CrossRef
40. Mock U, Nickolay L, Philip B et al. Automated manufacturing of chimeric antigen receptor T cells for adoptive immunotherapy using CliniMACS prodigy. Cytotherapy 2016; 18: 1002–11. CrossRef
41. Sukumaran S, Watanabe N, Bajgain P et al. Enhancing the Potency and Specificity of Engineered T cells for Cancer Treatment. Cancer Discov. 2018; 8: 972–87. CrossRef
42. Cherkassky L, Morello A, Villena-Vargas J et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Invest. 2016; 126: 3130–44. CrossRef
43. Avanzi MP, Yeku O, Li X et al. Engineered Tumor-Targeted T cells Mediate Enhanced Anti-Tumor Efficacy Both Directly and through Activation of the Endogenous Immune System. Cell Rep. 2018; 23: 2130–41. CrossRef
44. Chmielewski M, Abken H. CAR T cells Releasing IL-18 Convert to T-Bet(high) FoxO1(low) Effectors that Exhibit Augmented Activity against Advanced Solid Tumors. Cell Rep. 2017; 21: 3205–19. CrossRef
45. Hu B, Ren J, Luo Y et al. Augmentation of Antitumor Immunity by Human and Mouse CAR T cells Secreting IL-18. Cell Rep. 2017; 20: 3025–33. CrossRef
46. Paulussen M, Craft AW, Lewis I et al. Results of the EICESS-92 study: Two randomized trials of Ewing’s sarcoma treatment – Cyclophosphamide compared with ifosfamide in standard-risk patients and assessment of benefit of etoposide added to standard treatment in high-risk patients. J. Clin. Oncol. 2008; 26: 4385–93. CrossRef
Affiliations
Prof. Dr. Claudia Rössig
University Children´s Hospital Muenster
Pediatric Hematology and Oncology
Albert-Schweitzer-Campus 1, Muenster, Germany