Lentiviral vector manufacturing process enhancement utilizing TFDF™ technology
Cell & Gene Therapy Insights 2020; 6(3), 455– 467
10.18609/cgti.2020.053
Oxford Biomedica (OXB) is a leading gene and cell therapy company that focusses on Lentiviral Vector (LV) technology innovation, with over 22 years of experience in process development and manufacturing. To address increased LV supply demand, OXB transitioned a GMP LV cell culture manufacturing platform from a cell factory-based adherent cell process to a more scalable, serum-free suspension process performed in single use bioreactors, scaled-up to 200 L in volume at the current time. The relative sensitivity of lentiviral vectors to environmental pH, salt concentration and shear stress during vector harvest and downstream processing continues to present a challenge for the development of efficient manufacturing processes [1]. This work evaluates the TFDF ™ (Tangential Flow Depth Filtration) technology developed by Repligen Corporation (Repligen) for the harvest of lentiviral vectors from cell culture supernatant from the bioreactor. The TFDF™ technology effectively separated cells and cell debris from vector particles. Harvest yields typically exceeded 90% with flux rates between 700–750 liters/m2/hour (LMH) at both 5 and 50 L scales. The tangential mode of TFDF™ was found to be sufficiently gentle on the cells to support multiple harvests, opening the possibility of greatly increasing LV vector production in a perfusion mode
The challenges of scaling up lentiviral vector manufacturing
Gene therapy utilizing lentiviral vectors to introduce therapeutic transgenes into the cells of patients represents a unique treatment option for an ever increasing range of genetic and acquired diseases. Key clinical successes, an increase in clinical trial activity in the space, and the approval of lentiviral vector based Advanced Therapy Medicinal Products collectively result in a dramatic increase in global demand for manufacturing capability [2]. However, development of a manufacturing process capable of generating lentiviral products with suitable quality attributes and at the capacity and cost of goods required to provide security of GMP supply remains a challenge across the biotechnology industry. OXB has been a pioneer in the development of products based on lentiviral vectors and has developed a commercial lentiviral manufacturing platform using transient transfection of mammalian cells grown in a serum-free suspension culture. During production, mature vector particles are secreted into the supernatant requiring physical separation of the vector from production cells and cell debris for vector harvest. This initial clarification step is typically performed via normal flow filtration methodologies utilizing filters appropriately sized to enable efficient transmission of vector whilst retaining cells and cell debris. Depth filtration based processes have shown variable recovery and can compromise product quality due to exposure of either the cells or the vector to excessive hydrodynamic stresses [3,4].
Tangential Flow Depth Filtration: a new technology for the isolation of lentiviral vectors
Repligen recently developed a novel filtration technology, tangential flow depth filtration (TFDF™) based upon passing a cell culture feed stream through a tubular depth filter in tangential mode (Figure 1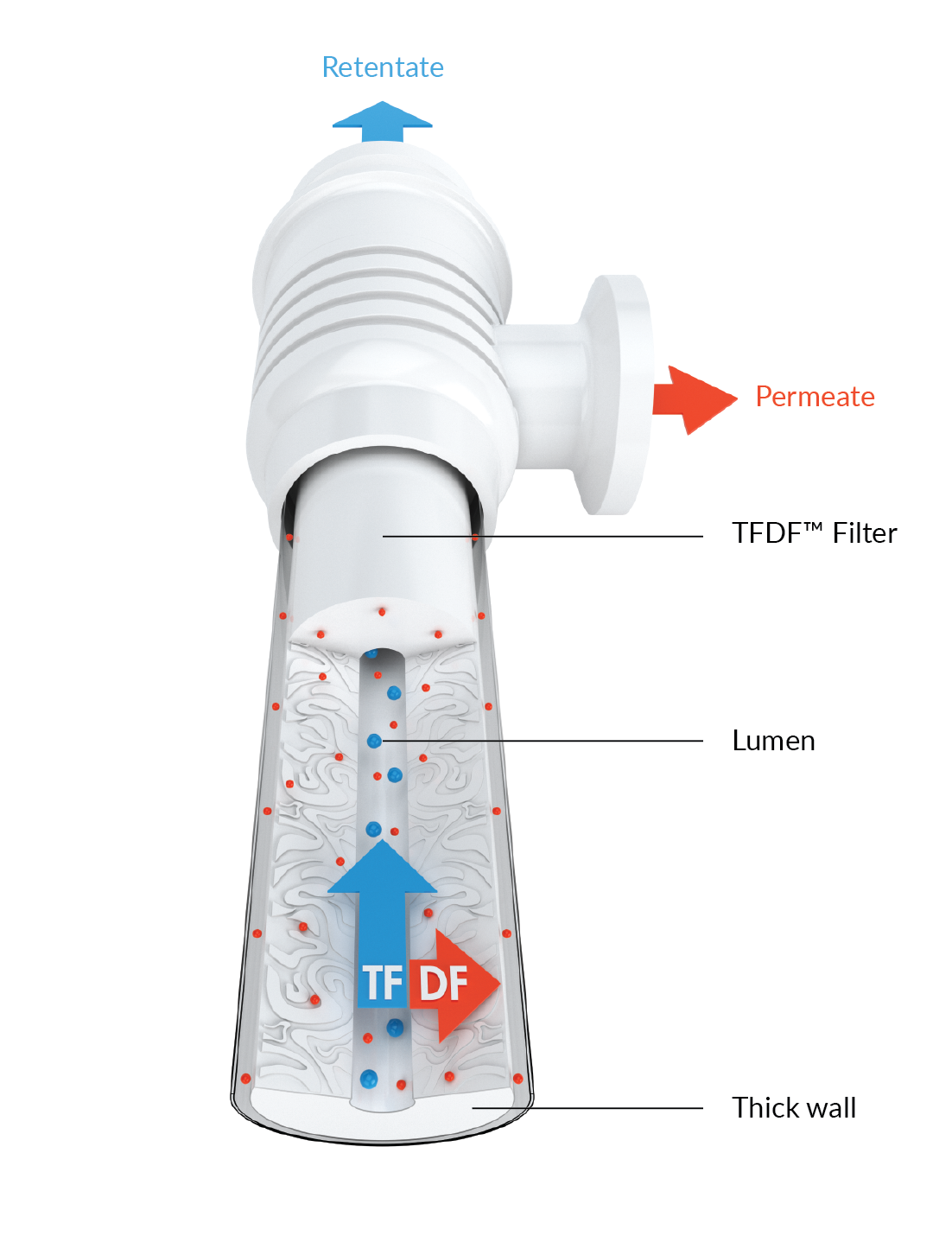
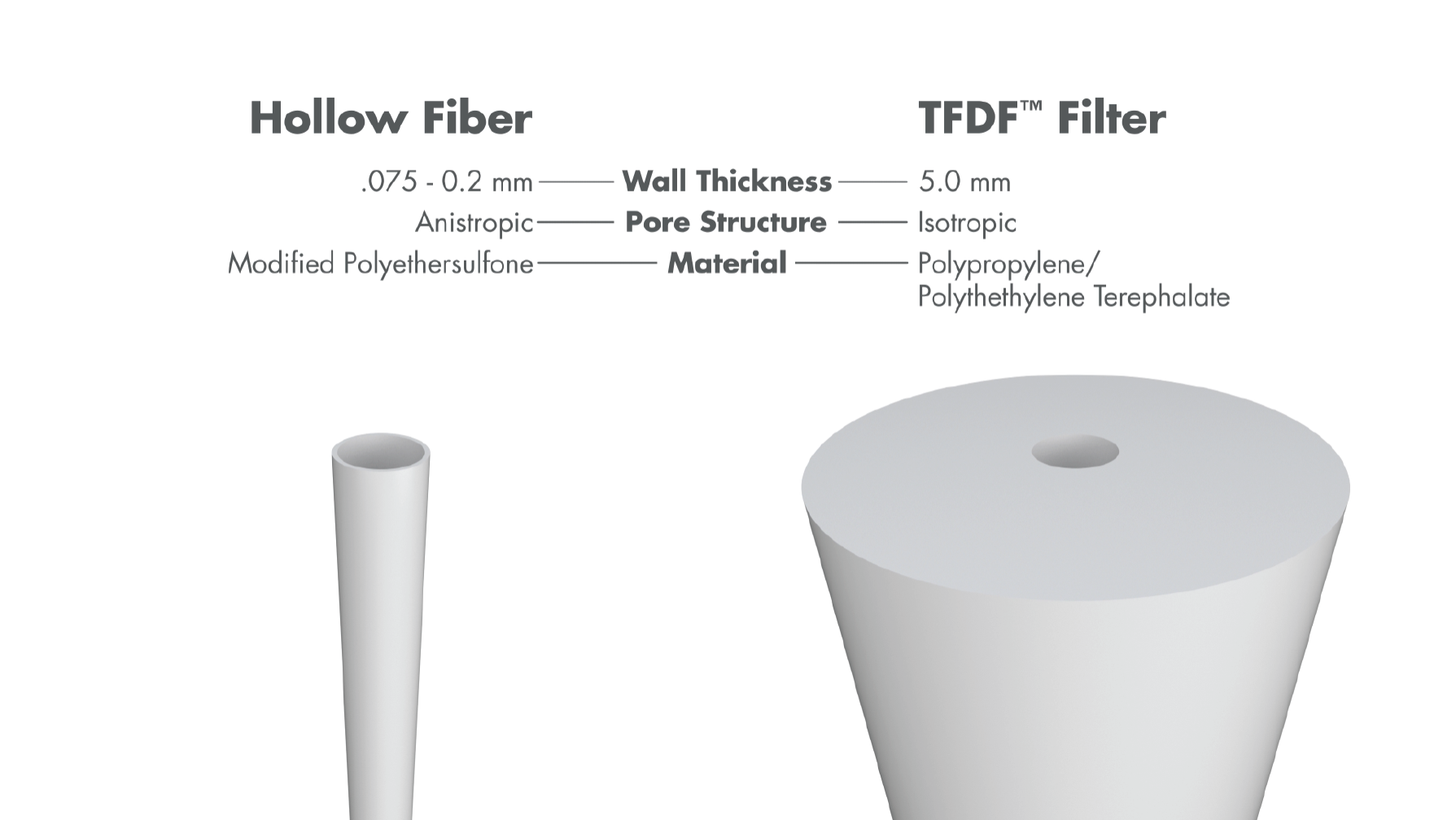
Attributes of the TFDF™ technology
The TFDF™ technology leverages aspects of both tangential flow (TF) and depth filtration (DF). Tangential flow through tubular depth filter supports high densities while the structure and capacity of the depth filter enable high product transmission (Figure 3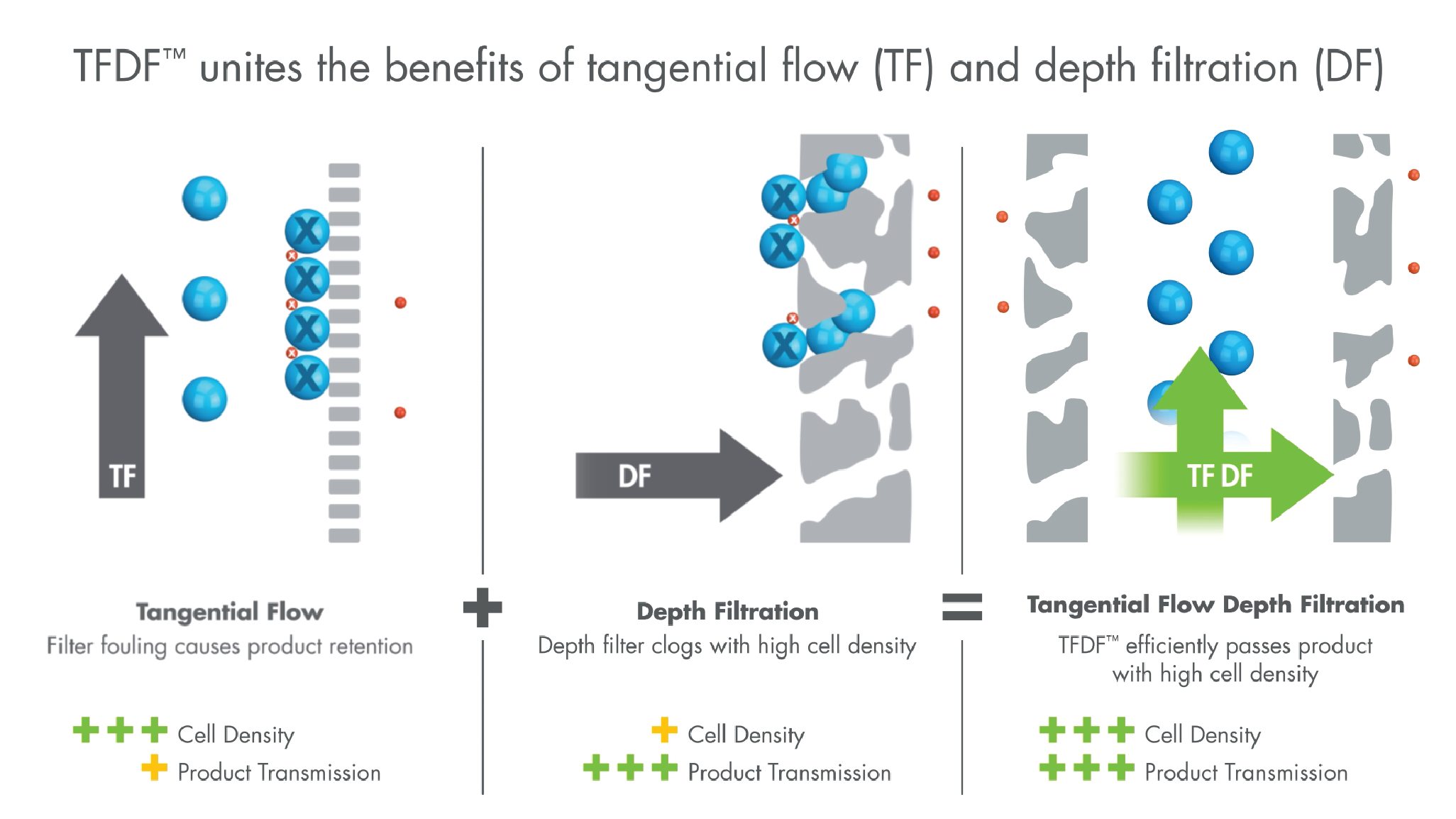
Tangential Flow Depth Filtration (TFDF™) System
The TFDF™ technology comprises hardware, software and ProConnex® TFDF™ flow path components. Both the hardware/software systems and the ProConnex® flow paths have been designed to support bioreactor volumes ranging from 1 to 2000 L (Figure 4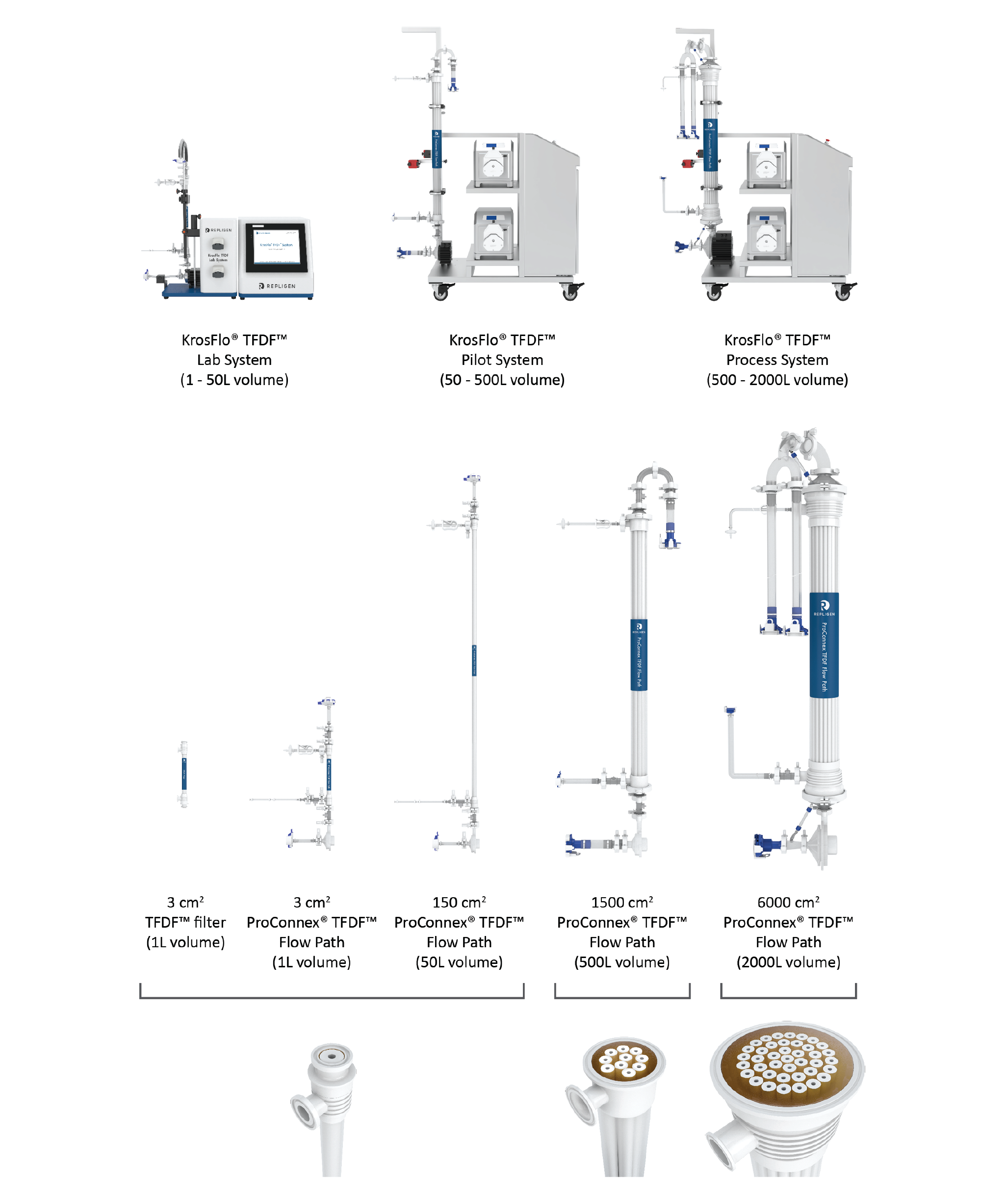
KrosFlo® TFDF™ evaluation methodology
Experiment #1: harvest of HIV-GFP in batch & perfusion modes
In order to assess the potential use of TFDF™ technology for the isolation of lentiviral vectors during manufacture in serum-free suspension culture, initial studies were performed using a Human Immunodeficiency Virus (HIV-1) vector expressing the Green Fluorescent Protein (GFP) reporter transgene and pseudotyped with the vesicular stomatitis virus G protein (VSV-G). Vector was produced at small-scale in a glass 5 L stirred tank bioreactor (STR) via transient transfection of mammalian cells using lentiviral expression plasmids complexed to a lipid based transfection reagent. To enable harvest of vector via the KrosFlo® TFDF™ System, a ProConnex® TFDF™ flow path with a filter surface area of 55 cm2 was connected to two in situ bioreactor dip-tubes using AseptiQuik® G connectors such that the bioreactor feed and retentate dip-tubes were coupled to their respective lines on the ProConnex® TFDF™ flow path. In a fashion distinct from a hollow fiber tangential flow filtration (TFF) process, the KrosFlo® TFDF™ System applies a pump to the permeate line and during vector harvest the flux for the permeate line was set at 700–750 LMH, a recommended starting point for initial process studies. Following harvest of vector containing supernatant, production cells retained within the bioreactor were re-suspended in fresh media and cultured for an additional period in order to assess the potential for performing multiple vector harvests from a single transient production process. A second vector harvest was performed using the same TFDF™ filter used for initial harvest. In order to assess the efficiency of the TFDF™ harvest process and the impact of TFDF™ harvest on lentiviral functionality, samples of vector containing supernatant were removed from the bioreactor immediately prior to each harvest and also from harvest material collected via the TFDF™ membrane. Functional vector titer in all samples was determined following transduction of adherently grown Human Embryonic Kidney (HEK) 293T cells with transduced (GFP positive) cells quantified using flow cytometry. Briefly, 72 h after viral transduction, treated cells were detached from the assay plate and analyzed for GFP fluorescence using a BD FACSVerse™ flow cytometer in conjunction with BD FACSuite™ software. Size and fluorescence data was collected for 10,000 individual events per sample and all samples were analyzed in duplicate. Appropriate analytical gating was utilized to identify the percentage of HEK293T cells that exceeded a fluorescence threshold determined by the background fluorescence measured in non-transduced cells. Functional vector titer was calculated assuming a single transducing unit per GFP positive cell.
Experiment #2: harvest of therapeutic lentiviral vectors
Whilst studies performed utilizing vectors expressing reporter genes such as GFP can be hugely informative for process development, the gene of interest encoded within the lentiviral vector construct can significantly impact both upstream and downstream process performance. Consequently, it is essential that any process development activities are assessed in the context of a directly relevant vector construct. In order to assess the performance of the KrosFlo® TFDF™ System for the harvest of clinically relevant lentiviral vectors, two 5 L STRs were utilized for the production of an Equine Immune Anaemia Virus (EIAV) based lentiviral vector expressing a therapeutic transgene pseudotyped with VSV-G. In one bioreactor, a single vector harvest was performed utilizing the KrosFlo® TFDF™ System operating with an applied permeate flux of 750 LMH. In the second bioreactor, vector was harvested utilizing a commercially available depth filter commonly used for clarification of material derived from mammalian cells grown in suspension culture. Samples were removed from both bioreactors prior to harvest and also from the respective clarified harvest material. Functional vector titer in all samples was determined following transduction of adherently grown HEK 293T cells with transduced cells identified by immunostaining and subsequent quantification using flow cytometry. Titer determination was performed as described above but transduced cells were identified by flow cytometry following treatment with a fluorescently labelled antibody conjugate targeting the therapeutic protein.
Experiment #3: increased process scale from 5 to 50 L
To evaluate the performance of the KrosFlo® TFDF™ System at a scale more applicable for the clinical manufacture of lentiviral vectors, production and clarification of an EIAV vector incorporating a therapeutic transgene was performed following transient transfection of mammalian cells in a 50 L Single Use Bioreactor (SUB). All vector production process operations were scaled in accordance with accepted engineering principles and in order to accommodate the larger process volume a ProConnex® TFDF™ flow path with a filter surface area of 450 cm2 was utilized at a permeate flux of 750 LMH for vector harvest. Samples were removed from the bioreactor prior to clarification and also from harvest material collected immediately following clarification through the TFDF™ membrane. Functional vector titer in all samples was determined as described above.
Tangential Flow Depth Filtration: a new modality for manufacturing lentiviral vectors
This study assessed the feasibility of the TFDF™ technology to harvest lentiviral vector particles in both batch and perfusion modes from serum-free, suspension cell culture. Performance of the TFDF™ technology was compared to a depth filtration method commonly used for clarification of viral supernatant. Experiments were scaled from 5 to 50 L.
Initial evaluation of the TFDF™ technology demonstrated clear separation of HIV-GFP lentiviral particles from production cells and cell debris during the harvest process. Analysis of the functional titer in Experiment 1 indicated successful recovery of vector through the TFDF™ membrane at the first harvest and also following a second harvest (Figure 5A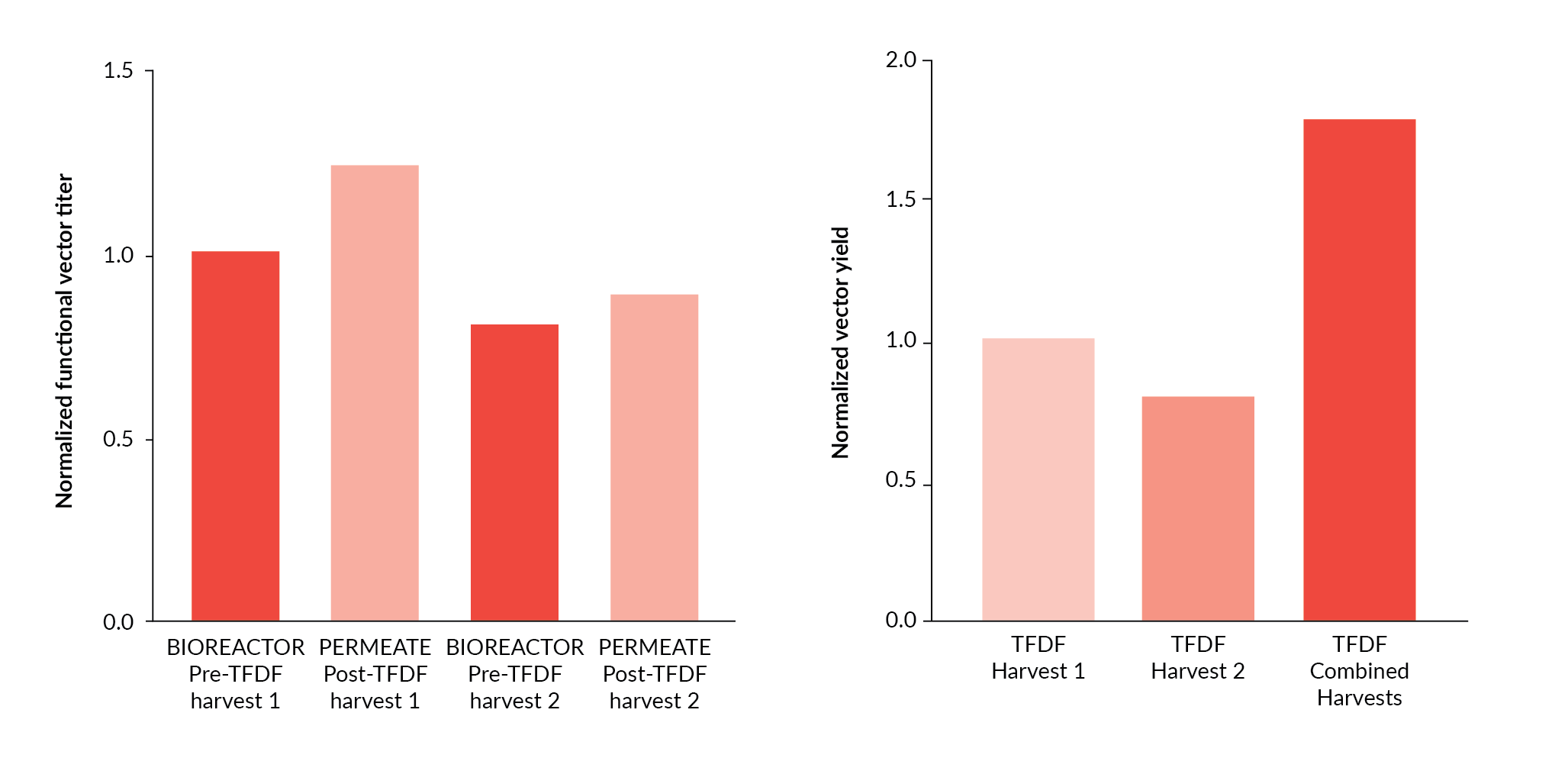
The potential benefits of TFDF™ technology for increasing overall lentiviral vector process yields was demonstrated by the ability to perform multiple vector harvests from a single transient transfection process. Although the observed vector titer in harvest 2 samples was lower than that observed in harvest 1 samples (Figure 5A), pooling of TFDF™ permeate material from both harvests resulted in an increase in overall process yield of approximately 80% compared to the single harvest process (Figure 5B). Multiple harvest steps are not feasible when primary vector clarification is performed using standard depth filtration approaches since the filters result in trapping of the cells outside of the production environment.
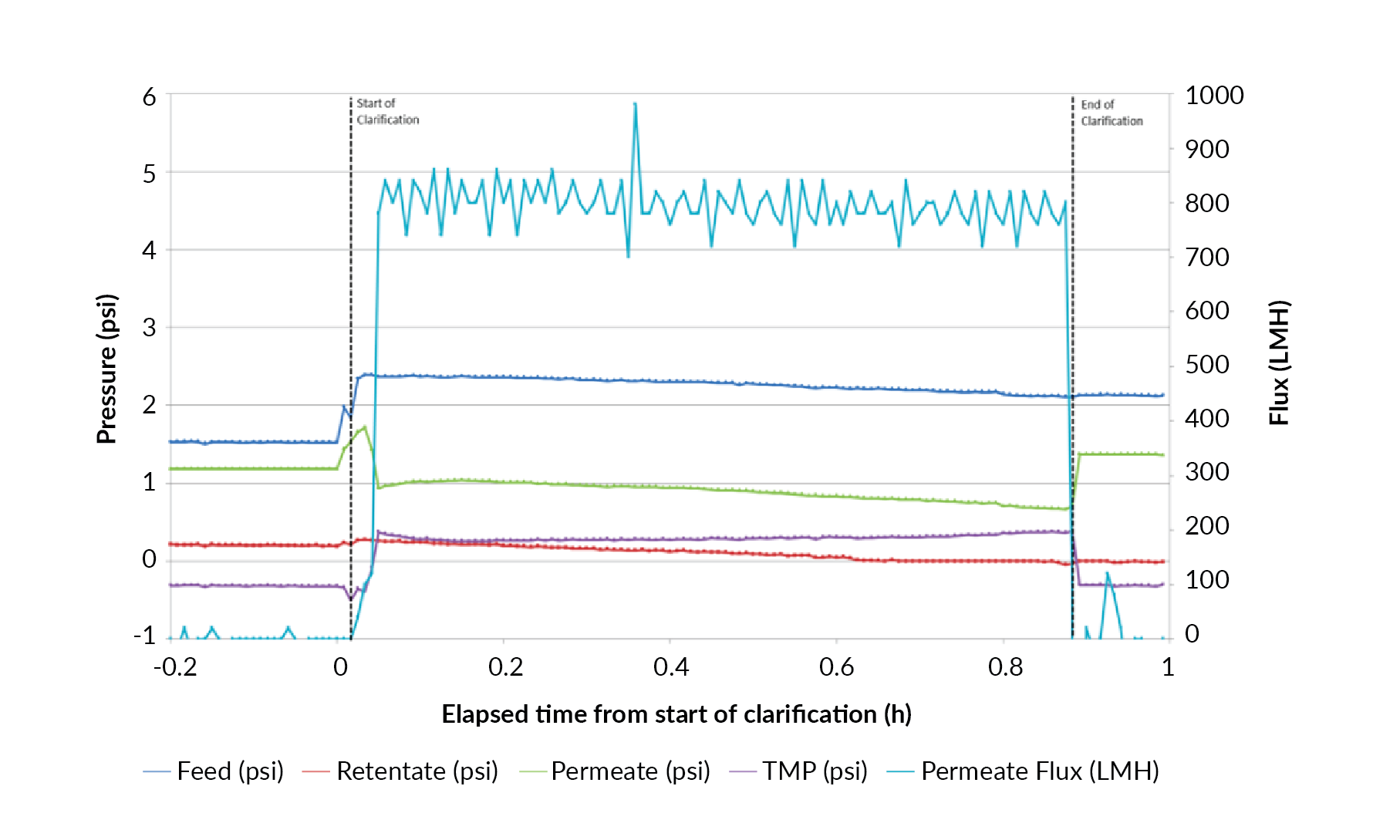
Harvest via the TFDF™ system was executed at a relatively high flux of 750 LMH with little evidence of membrane fouling. The flux profile as a function of time indicates relatively minor pressure changes in the feed, retentate, permeate and transmembrane pressures (TMP) over the course of the one hour unit operation (Figure 6A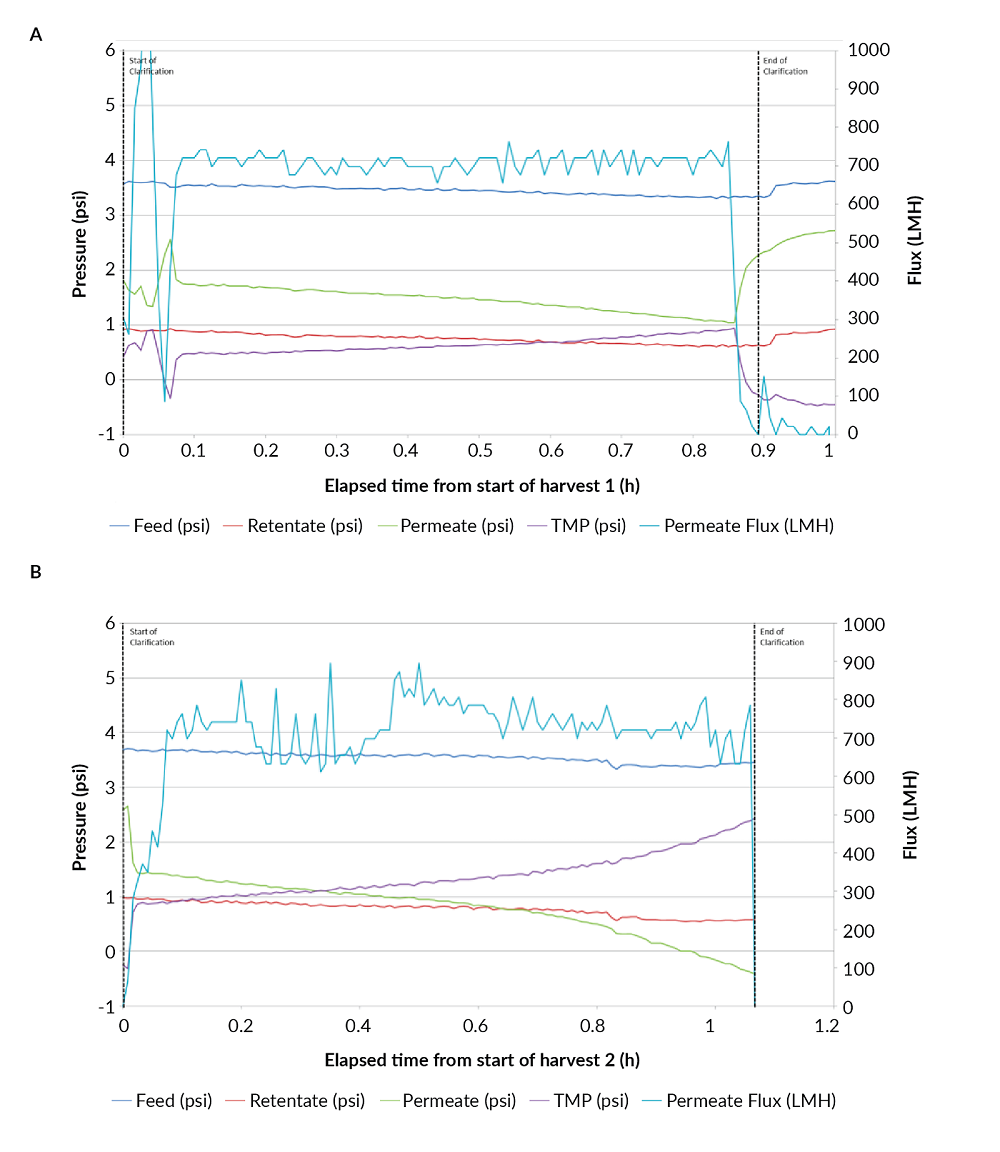
A similar flux profile was generated during harvest 2 using the same TFDF™ filter as harvest 1 (Figure 6B). Flux was again maintained at 750 LMH for approximately 45 minutes. Feed and retentate pressures remained stable with less than ±1 psi variation. TMP started at approximately 1 psi and increased by only 1.5 psi to a final value of 2.5 psi, indicating that while some cells and cell debris restricted flow through the membrane, the amount did not impact flux.
In order to extend the TFDF™ evaluation to include a clinically relevant vector, lentiviral vector harvest utilizing the TFDF™ system was repeated utilizing an EIAV based lentiviral system incorporating a therapeutic transgene. In three independent studies, vector was produced in 5 L STRs and harvested utilizing TFDF™ technology (Figure 7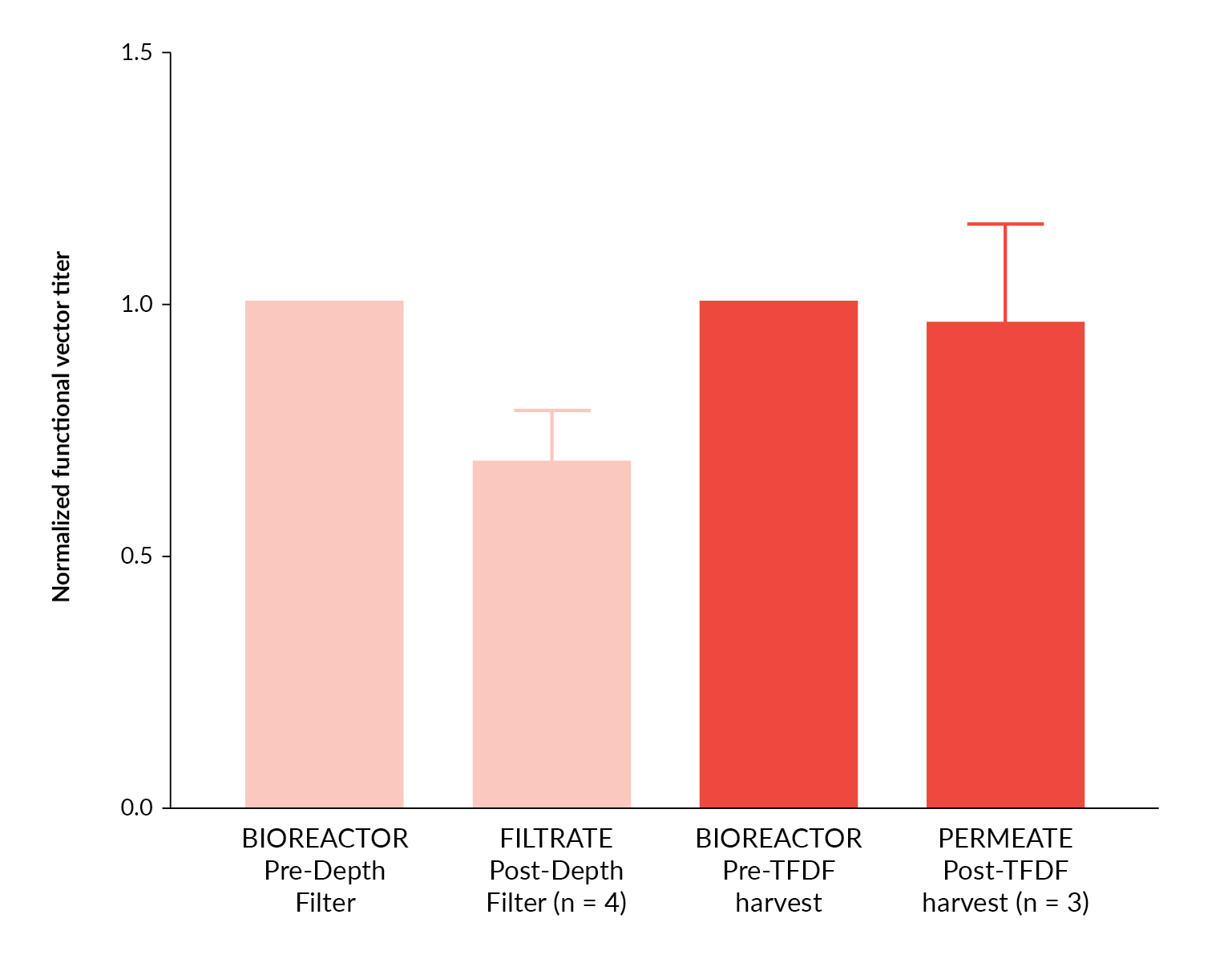
Overall, these data demonstrate the efficient recovery of lentiviral vectors utilizing TFDF™ technology and application of a multiple harvest approach could potentially be utilized for further increases in process yield.
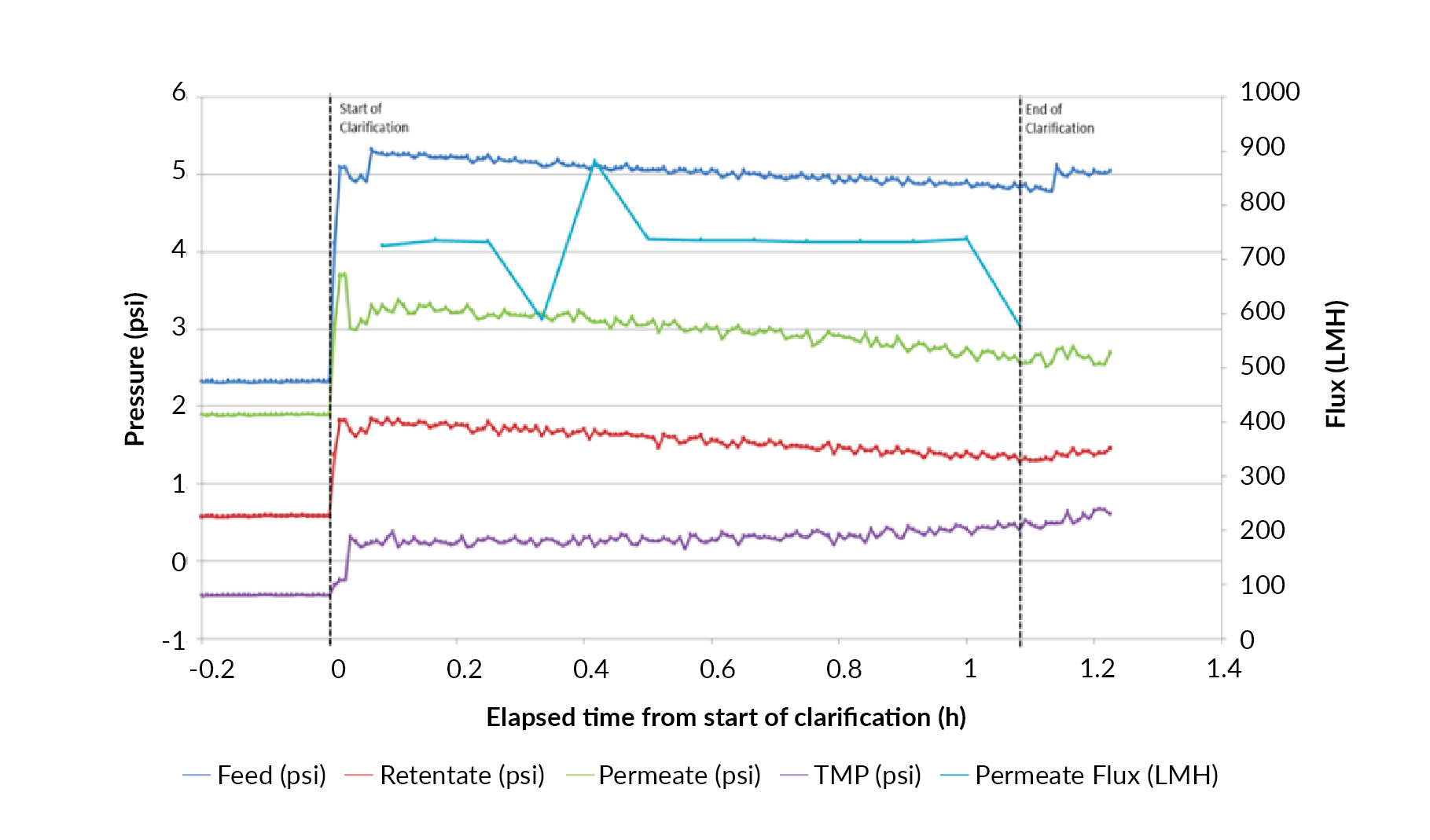
Process scalability represents a critical requirement of manufacturing technologies. Harvest clarification of an EIAV lentiviral vector expressing a therapeutic transgene was therefore scaled ten-fold from 5 to 50 L in a single-use bioreactor. Vector recovery was assessed in three independent bioreactor studies (Figure 9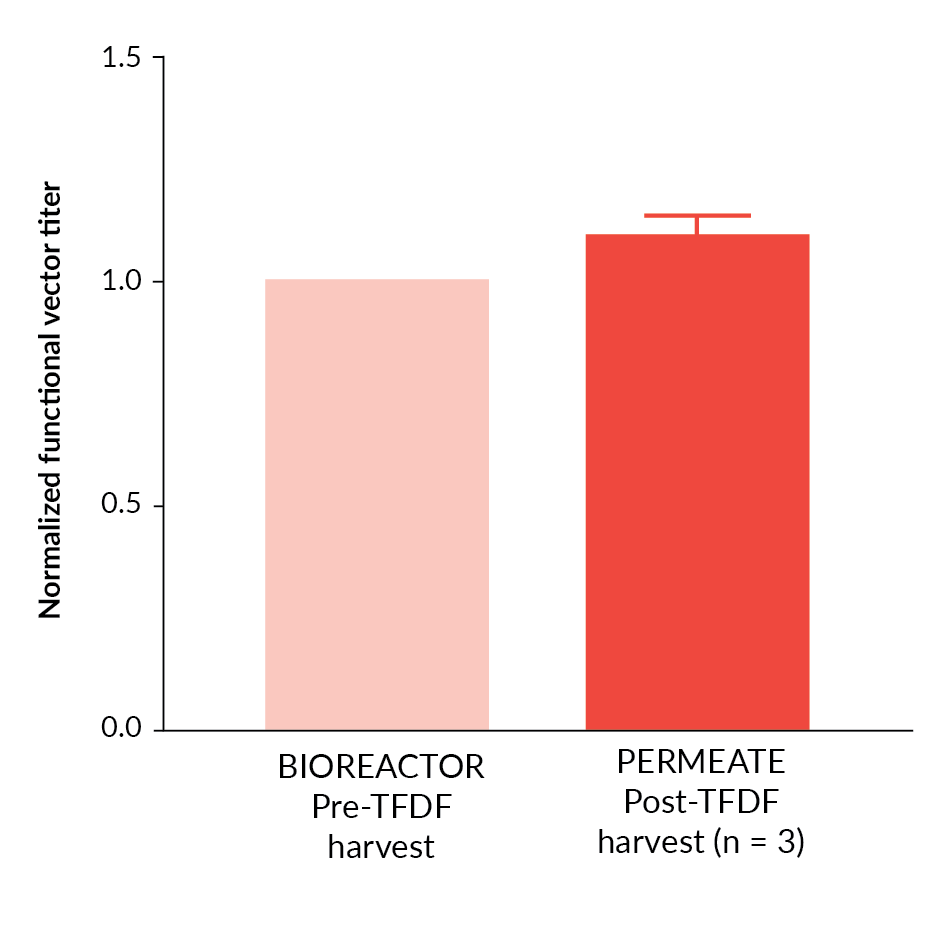
Towards a perfusion mode?
This study evaluated the TFDF™ filtration technology developed by Repligen for separation of cells and cell debris from lentiviral vectors as a means of harvest clarification in both batch and perfusion mode at scales between 5 and 50 L. This study demonstrates the first practical demonstration and application of this approach for the clarification of lentiviral vector material prior to further downstream purification using the effective 2–5 µm pore rating of the TFDF™ filter. The clarification unit operation was completed in less than an hour, enabled by flux rates between 700–750 LMH, with yields typically greater than 90%. Comparable results were found for both HIV and EIAV based lentiviral vectors and for vectors expressing a reporter gene as well as a therapeutically relevant transgene.
Whilst the high process flux and vector yields observed using TFDF™ mediated harvest clarification offer significant improvements over standard clarification approaches, extension of the TFDF™ to support a perfusion mode for lentiviral vector production holds the potential to significantly increase productivity. Using the HIV-GFP vector, use of TFDF™ as a cell retention device for perfusion of vector particles was shown to be successful with inclusion of a second harvest resulting in an overall increase in process yield of approximately 80%.
Setup and operation of the TFDF™ technology was technically simple, facilitated by the integration of pressure sensors, TFDF™ filter, tubing and clamps into a single ProConnex® flow path, requiring less than 30 minutes for consumable installation and filter priming prior to the run. Users with knowledge of hollow fiber or flat sheet methods will find the overall user experience to be familiar.
To conclude, the KrosFlo® TFDF™ system was found to be an effective tool for lentiviral vector harvest clarification. The process is efficient, easy to use, scalable and offers the potential to improve overall vector yields via repeated harvesting from the production vessel.
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: M Bransby has a patent, US10538727B2.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2020 Oxford Biomedica (UK) Ltd and Repligen Corportion. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Revised manuscript received: Apr 14 2020. Publication date: Apr 28 2020.
References
1. Merten OW, Hebben M, Bovolenta C. Production of lentiviral vectors. Mol. Ther. Methods Clin. Dev. 2016; 3: 16017. CrossRef
2. ARM Q3 2019 data report: Website
3. Moss D. Vector purification: issues and challenges with currently available technologies. Cell Gene Ther. Ins. 2019; 5(9): 1125–32. CrossRef
4. Raghavan B, Collins M, Walls S et al. Optimizing the clarification of industrial scale viral vector culture for gene therapy. Cell Gene Ther. Ins. 2019; 5(9): 1311–22. CrossRef
5. Geraerts M, Willems S, Baekelandt V ,em>et al. Comparison of lentiviral vector titration methods. BMC Biotechnol. 2006; 6: 34. CrossRef
Affiliations
Thomas Williams
Oxford Biomedica, Windrush Court, Transport Way, Oxford OX4 6LT, UK
Oliver Goodyear
Author for correspondence:
Oxford Biomedica, Windrush Court, Transport Way, Oxford OX4 6LT, UK
Lee Davies
Oxford Biomedica, Windrush Court, Transport Way, Oxford OX4 6LT, UK
Carol Knevelman
Oxford Biomedica, Windrush Court, Transport Way, Oxford OX4 6LT, UK
Michael Bransby
Repligen Corporation, 41 Seyon Street, Waltham, MA 02453, USA
Kyriacos Mitrophanous
Oxford Biomedica, Windrush Court, Transport Way, Oxford OX4 6LT, UK
James Miskin
Oxford Biomedica, Windrush Court, Transport Way, Oxford OX4 6LT, UK