Broadly-applicable imaging platforms are necessary for optimizing cell therapies in solid tumors
Cell & Gene Therapy Insights 2019; 5(7), 629-638.
10.18609/cgti.2019.071
The introduction of immunotherapy, particularly immune cell therapies, have transformed the therapeutic landscape in recent years. Cell therapies are now finally reaching patients in growing numbers, with many more set to come through the pipeline in the next few years. In 2017, Novartis’s Kymriah® received FDA approval for patients up to 25 years with acute lymphoblastic leukemia (ALL) that has either relapsed or is refractory. This approval followed the near extraordinary results of the case of Emily Whitehead, a 7-year-old girl who had been fighting ALL for more than a year, whose cancer went in to remission following treatment. Emily is now 6 years cancer-free. In the months that followed Kymriah’s® approval, two other gene therapies joined the market: Yescarta®, a CAR T-cell therapy marketed by Gilead to treat certain types of large B-cell lymphoma; and Luxturna®, Spark Therapeutics’ gene therapy for a rare form of inherited vision loss. Although these three therapies would be the first, they will certainly not be the last, with over 900 cell and gene therapy-related clinical trials in planning phase or ongoing, according to www.ClinicalTrials.gov. Following the success of Kymriah®, all eyes now turn towards large patient groups with solid tumor cancers and whether the success of CAR-Ts can be reproduced in these patients. However, solid tumors represent a much greater challenge. These tumors incorporate mechanisms designed to keep T cells out, due to the tumor microenvironment where any cell and gene therapy would need to incorporate a strategy to overcome the tumor’s blocking mechanism. To date, a strategy for this simply does not exist, and before any such strategy can be comprehensively evaluated in vivo, a means to track these cells in situ and subsequently monitor their success is fundamental. This strategy could develop a first in class prognostic indicator that can follow the localization or proliferation of these cells. Stratifying responders versus non-responders for cell therapies could be achieved at the clinic through effective cell tracking as the healthcare industry now embraces the clear clinical benefits of the personalized medicine approach. For the clinician and scientist to understand these cell therapies in greater detail, we need to measure the function and behavior of these cells when delivered to the patient. What are the cells doing? Where do they go after being administered to the patient? This commentary piece focuses on the pressing need to develop a broadly applicable platform that incorporates in vivo imaging and modelling for the optimization of cell therapies, that can be integrated in existing cell therapy supply chains. This platform requires a collaboration of the imager, the preclinical scientist, the regulator, manufacturer and the clinician to make it functional for optimizing the cell therapies in solid tumors. If successful, the platform should be robust enough to be rolled out at any clinical site and be adaptable to any cell therapy type.
The Challenges of Novel Cell Therapies for Solid Tumors
Cell therapies, in general, enter a ‘black box’ once they are transferred to the patient. It is extremely difficult to obtain direct information on their viability, localization, numbers and functionality in a longitudinal and kinetic manner. This is in addition to several unknowns in their fundamental mechanism of action, and the effect of combinatorial treatments. Firstly, let’s address 1) CAR T cell biodistribution. T-cell therapies are typically delivered intravenously, and sometimes in more than one infusion. The cells distribute rapidly through the bloodstream, reaching tumor sites and more often than not, irrelevant sites [1]. This can lead to off-tumor on-target or off-target effects which is a major safety issue for patients [2], even resulting in death [3]. Current gold standards to assess cell biodistribution preclinically involve time-consuming necropsy and histopathological staining of slices of a small fraction of the tumor tissue, which is prone to sampling error in addition to standard qPCR. Flow cytometry and bioluminescence imaging with luciferase as a reporter gene are also commonly used [4]. The former, however, suffers the limitation of being quantitative only on blood samples or at the time of necropsy, while bioluminescence imaging is not applicable to humans. Therefore, developing a rapid and quantitative preclinical technique for screening cell biodistribution and survival would be highly useful. While some studies have used SPECT with radioactive Indium tracers to localize transplanted T cells [5], CAR T cells have so far only been rarely imaged, and typically by the use of PET reporter genes [6]. While this is a very specific technique, it has some drawbacks: it exposes therapeutic cells to radioactivity, which can lead to undesirable effects; the reporter gene can become immunogenic; quantification of cell numbers remains complicated due to variability of tracer uptake in vivo; regulatory clearance must be obtained for each radiotracer; clinical applicability would be limited to the larger centers that can generate the specialized radiotracers. In preclinical systems, bioluminescence imaging with luciferase as a reporter gene is more commonly used [7]. Generally, no single imaging modality to date can satisfy all the requirements for the types of information needed. Secondly, what about 2) cell numbers at the tumor? The numbers of therapeutic cells that reach the tumor(s) [8], their kinetics, dose(s) and delivery, to obtain clinically relevant responses are a major determinant of potential success. T-cell infiltration to tumors can be a key limiting factor for the success of immunotherapy [9]. A direct quantitative assessment of the cells reaching the tumor would therefore provide a significant advantage to the field. Currently, the best solution for this would be either a radiotracer or an emerging technique like 19F MRI, which is also quantitative. Radiolabels are less suitable due to their shorter half-lives and radioactivity [10] burden, which many clinical centers are not equipped to handle effectively. Even with newer tracers based on longer-lived isotopes, such as zirconium-89 (around 3 days half-life), this is still insufficient time, as cells are typically generated and labelled off-site before being frozen for storage and transport to the clinical site; in addition to higher costs, administrative and safety burden, and ‘hassle’ in dealing with radioactivity. In addition, many of the CAR T cells will also take several days to weeks to expand and proliferate once clones are activated from encountering the target receptor Approaches such as 19F MRI would allow for quantification without the limitations of ionizing radiation, although the technique is less well-established and less sensitive. While there is a clear link between the number of therapeutic cells that reach the site and efficacy, another essential factor is their functional status, which includes 3) the proliferation of T cells at the tumor site. Functional T cells are expected to proliferate rapidly upon encountering the antigen that they have been programmed to recognize. This is a key premise of CAR T cell therapy [11]. Thus, we need to be able to assess T-cell proliferation at the site. In vivo assessment of proliferation is extremely difficult. So far, there have been only two publications using non-invasive, clinically-applicable or clinical techniques, both from the co-author of this commentary piece [12]. First, 19F MRI was used to assess antigen-specific T-cell recruitment to a relevant draining lymph node in a quantitative manner over a 3-week period in mice, taking proliferation into account. The second used 18F PET with an injectable PET tracer (FLT) to assess tumor-specific T-cell proliferation in lymph nodes in melanoma patients. Assessment of proliferation of T cells at a tumor site is complicated by the fact that tumor cells will also be dividing in an unpredictable manner. As a result, novel combination strategies such as utilizing an internal label in the T cells (e.g., 19F nanoparticles) [13,14] and an injectable PET tracer for proliferation could be a successful approach. Both the 19F and 18F signals are quantitative, leading to metrics for each patient. The relationship between the two values, actual T-cell numbers, and actual T-cell proliferation is expected to be complex and heavily interlinked. Therefore, as part of such a platform, modelling would be integral to understand this relationship, to develop a single indicator from all these data as a measure of T-cell activity in a patient. Finally, 4) tumor infiltration – whether the T cells are able to penetrate the tumor or whether they remain localized to the tumor periphery [15] is another essential determinant of CAR T cell therapy success. Different tumor types can also present barriers of different degrees. This can readily be studied in preclinical models using histology on extracted tumors or biopsies. In humans, non-invasive imaging would be much more feasible. Readily available MRI provides excellent soft tissue contrast, allowing visualization of the tumor. In addition, more sophisticated techniques such as tumor blood flow, the presence of a necrotic core, volume, and other multiparametric MRI techniques can be assessed using standard clinical workflows. These can be combined with specific 19F MRI of the labelled T cells to assess localization with the tumor. Understanding the challenges that cell therapies face in solid tumors has fueled the development of novel imaging approaches to track immune cells in vivo and in the patient in clinical trials [16]. Understanding the dosage required to elicit the prescribed response is also key as we want to limit over dosing and possible toxicity resulting from cytokine release syndrome or simply not dosing high enough to be effective. Multiple imaging modalities are being investigated by many groups worldwide, but to succeed, a broadly applicable platform that can be rolled out at multiple clinical sites, displaying ease of use and integration into existing cell therapy supply chains must be considered.
Non-invasive Imaging Solutions
One potential solution currently under development is to apply a customizable imaging agent with multimodal imaging capabilities together with computer modelling to provide an early stage prognostic indicator for each patient that can be applicable to clinical CAR T therapy. A multimodal imaging agent would allow the use of different imaging techniques, and thus the acquisition of different kinds of information as needed. Furthermore, because the same agent could be used for different modalities, the regulatory burden would be reduced. This will also greatly reduce cost and enhance commercial viability. However, the multimodality must not come at the cost of performance for any single modality. One example is the agent developed by Cenya Imaging that is currently already produced at GMP-grade for clinical cell tracking of therapeutic dendritic cells (DCs) in melanoma patients. The nanoparticle agent is visible in 19F MRI, ultrasound, photoacoustics and fluorescence imaging; a mix of established and emerging imaging modalities, both clinical and preclinical. Preliminary analyses suggest that these nanoparticles could be approved as a generic ‘cell labelling’ agent for ex vivo cell labelling before in vivo transfer of the cells. The nanoparticles consist of a perfluorocarbon (PFC) entrapped in poly (lactic- co -glycolic) acid (PLGA). The choice of PFC, diameter, additional content (fluorescent dye, drug), and surface charge or coating (targeting ligand) can be varied. The nanoparticles are suitable for 19F MRI, fluorescence, ultrasound and photoacoustic imaging; variations of the GMP-grade particle will be tested in the preclinical studies as needed at GLP-grade, such as surface functionalization and radiolabeling. Multimodal imaging approaches such as this are key as they can be utilized for the tracking of many types of immune cells allowing the platform to be adaptable to different cell therapies at different clinical sites utilizing standard imaging equipment.
The need for the Right Preclinical Models
To optimize therapeutic efficacy, in-depth, quantitative and qualitative knowledge of the T-cell distribution within the targeted tumor lesion, and of the number of cells needed to evoke a therapeutic response, is required. A strong preclinical arm to the platform is essential to directly enumerate tumor accumulation and amplification in various preclinical models and to correlate it with ‘pre-conditioning’ regimens including tumor debulking. The results from such preclinical research enables a better understanding on T-cell-tumor dynamics, generates suitable indicators of T-cell efficacy, and provides input for innovative clinical studies. Physiologically relevant murine models of solid tumors have to be adopted. The right preclinical model to elucidate the mechanism of cell therapies, however, remains a major challenge in the advanced therapies space and represents one of the major bottlenecks in the cell therapy development pipeline. The availability of the ‘right’ preclinical models can help validate the potency for example of 19F-labeled T cells and solve present bottlenecks to effective therapeutic responses. A system that is capable of correlating in vivo animal results with in vivo clinical results is of high interest. Given that (i) most/all animal models are considered to not be relevant species for PK and toxicology assessments, especially for CAR T cells; and (ii) that human clinical biodistribution using many techniques applied to animals is not feasible (i.e., necropsy, luciferase marking, etc.). There is an important potential knowledge gap in the field that imaging techniques could vastly improve. For example, physiological differences between tissues and differences in target gene expression (e.g., CD19, CD30, etc.) and other gene expression may mean that biodistribution in animals does not predict what occurs in humans, but imaging may be able to identify correlates or aspects of the CAR design that may improve this issue. A robust platform to explore the use of non-invasive imaging in cell therapies must have the right preclinical component that can exploit multiparametric imaging modalities to answer critical pending issues in T-cell therapy of solid tumors (suppressive microenvironments, or unequal/suboptimal distribution of T cells), and to develop combined adaptive cell therapy approaches, suitable to ameliorate ACT efficacy for the treatment of solid tumors and metastasis.
The Regulatory Challenges to Combining Imaging & Cell Therapies
To complement the imager, clinician and the preclinical scientist, a fully functional platform must have a strong regulatory component. Any medicinal product and medical device must comply with various regulatory requirements before it can be authorized for use in the clinic. Guidance on what regulations to comply to and how to achieve this compliance is not always straightforward. This is especially in the case of innovative products such as the nanotechnology mentioned in the previous section, combining a medical treatment function with an imaging function and an advanced therapy medicinal product (ATMP). Thus, the relatively novel use and limited experience with cell therapies and nanomaterials for medical purposes, both by innovators and regulators, present a formidable barrier for the development of these technologies. Awareness of the applicable regulations from an early stage will facilitate the efficient development and application of regulatory compliant products, e.g. by anticipating requirements for quality control, efficacy and safety assessments; or by validating the generated data with the competent regulatory authorities. In particular, nanoparticles, especially those with imaging functions and when combined with advanced therapies, represent complicated borderline products (products in which the final regulatory classification is not immediately clear) with important regulatory concerns. The tracking and labelling of CAR T cells straddles a line between pure investigation, clinical diagnosis and also biomarker development each of which has a different regulatory connotation. For example, stratification of patients based on imaging results might require formal qualification procedures which may involve time, cost and relatively large patient pools which are not always available for late stage and/or orphan conditions.
As a result, Regulatory Bodies should be regularly consulted and involved in the activities in order to facilitate a bi-directional exchange of expertise and information and define clear pathways for such agents in the future. The chemistry, manufacturing and control (CMC) package, preclinical development plan and the first-in-human clinical trial design for novel imaging agents applied in the platform should be agreed with regulators – in the European context – via the conduct of early meetings at the EMA involving CAT members and Scientific Advice (SA) with a selected national competent authority (NCA). The competent authorities are responding to the demands of innovative and combined therapies with specially tailored early-phase meetings and also the new INTERACT (INitial Targeted Engagement for Regulatory Advice on CBER ProducTs) meetings with FDA. However, one important issue in the EU can be that medical devices are not assessed by the EMA or the competent authorities in the same way as medicinal products but are assessed by the Notified Bodies where scientific advice procedures are limited. Therefore, depending on the regulatory classification of the product or the components, different expertise may be required. As the technological and regulatory landscape evolve at different rates, the demands on expertise and experience can become difficult to navigate. These interactions illuminate the regulatory, technical and conceptual pathway to the clinical trial whereas discussion with NCAs can improve the evaluation and conduct of the clinical trial to ensure maximum safety and scientific validity. The clinical use of the innovative imaging technology constitutes an unprecedented advance beyond the state of the art, allowing to track the fate of cells after its use in cell therapy in human beings. The benefit of this innovative technology can be extended from the population suffering from solid tumors or any type of cancer or regenerative therapies in healing ‘incurable’ diseases.
Creating the Right Community to Develop the Platform
At the technological level, industries and health sector stakeholders such as hospitals need to be able to implement the labelling, modelling and standardization processes of a broadly applicable imaging platform to define better therapies and novel prognostic indicators for cancer, and several other diseases with limited treatment options. The platform could also deliver a prediction model, suitable to define efficacy at early treatment stages that could maximize the efficacy/cost effort allowing to spread ATMPs to larger patient groups thereby increasing health equality and social economical sustainability. The effective tracking of cells in the tumor can offer the clinician an early prognostic indicator as to whether the therapy has reached its target and is working as needed. Target groups will include the scientific and medical community, and patient populations. Biomedical and biotech companies, as well as Big Pharma and the national health care providers will also benefit from such a platform as the research activity will define quantitative parameters of therapy efficacy/optimization.
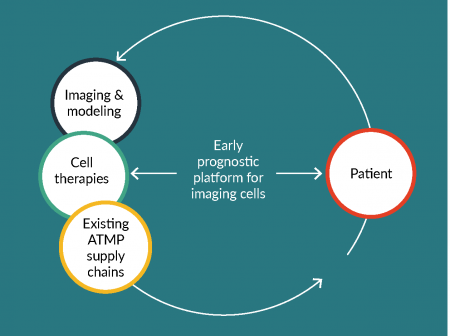
These groups will be key to creating a community focusing on cell tracking in cell therapy, offering the cell therapy developer a unique, centralized ‘one-stop shop’ to acquire the necessary expertise and support services that such a platform would offer. With the many clinical trials in CAR T therapy in solid tumors in progress, the issue of tracking these cells and their delivery will be pivotal. The establishment of the platform (Figure 1) will ensure this array of ‘must have’ services to support this therapeutic approach which could be made available to the scientific community. To develop such an innovative technology option in cell tracking for T-cell therapies, a number of partners with complementary activities must be involved to create a robust platform. European infrastructures such the European Infrastructure for Translational Medicine (EATRIS), which support researchers in developing their biomedical discoveries into novel translational tools and interventions could be one such partner. EATRIS consists of a network of academic institutions of excellence in translational research, based in 13 European countries. Of note EATRIS has over 40 institutions actively working in the ATMP space that can provide the pre-clinical arm and the expertise at the clinical level, with several sites already running CAR-T trials. EATRIS can be instrumental to create a two-way channel between internal and external stakeholders to work closely with all partners to develop a first of its kind Cell tracking Platform, providing a comprehensive catalogue of services that meet the demands of the cell therapy community, keeping the overarching aim of making CAR-T therapy in solid tumor patients a practical and economically viable therapeutic approach for the cancer patient. Such a Platform would be created from the knowledge and partnerships ranging from the preclinical innovation teams, the regulator, the manufacturer and supply chain, the imager and the clinician at the therapy administering clinic. Similarly, close interaction with global networks already established in the cell tracking space could collaborate with the Platform to broaden the knowledge base and facilitate exchange of best practices.
One such network of key opinion leaders in cell tracking and cell therapies is found at the Health AND Environmental Science Institute (HESI), which is an independent non-profit dedicated to bringing together global teams of scientists from academia, government, industry, and NGOs to solve the most pressing risk and safety challenges facing humans and the environment today. The research facilitated by HESI’s technical committees is designed to identify and test solutions that can be broadly applied. Some of the practical applications of HESI-directed research include improving patient safety, reducing the use of animals in testing, protecting the environment, and enhancing product safety. HESI is based in Washington DC, USA, but operates globally. HESI’s Cell Therapy – TRAcking, Circulation, & Safety (CT-TRACS) committee was launched in 2016 as an Emerging Issues sub-committee to identify key needs for assessing the safety of cell therapies and identify opportunities to meet these needs. This program provides a neutral platform for cell therapy developers, researchers, regulators, imaging specialists and other stakeholders to interact, discuss current challenges and identify best practices to ensure that these therapies are safe and effective for use. It brings together an international and multi-disciplinary team of experts with interest in sharing their knowledge, common challenges and seek consensus on finding harmonized solutions. In particular, the committee aims to bring awareness on how the application of existing cell tracking technologies, methods, and best practices can benefit the clinical translation of these new therapies. Since its inception, CT-TRACS has gathered more than 60 members from about 25 organizations across the United States, Europe and Japan. Altogether, EATRIS, HESI CT-TRACS, imaging agent developers such as Cenya and regulatory groups such as Asphalion, combine the knowledge base, access to international expert input in multiple fronts (researchers, tools developers, enabling technologies experts, regulatory experts, other professionals/end users), and a large network of academic institutions of excellence in translational research where tools and technologies needed for pre-clinical and clinical studies are available. These organizations are willing to come together to facilitate exchange of best practices and address challenges. In what shape or form a “Cell Tracking Hub” facilitating access to imaging platforms and solutions in support of the safe and successful translation of cell therapies remains to be determined, but key players across the globe are aligned in respect to the need for it.
Finally, we can never mention cell therapies without a word on possibly the biggest roadblock of all to the patient, the price. A global failure to make cell therapies available at realistic prices continues to block market-approved ATMPs from reaching the patient, or only lasting for a short period of time. Hence the collective work of this cell tracking platform must continue to address issues such as reimbursement in the hope of keeping prices realistic and by doing so ensuring that the therapy in question is an available option at the clinic. The ability to stratify patients early into responders versus non-responders at the clinical trial stage in addition to ascertaining minimum dose requirements will all help in reducing production and administrative costs and ultimately the price tag on these therapies. The early prognostic indicator that cell tracking can provide will promote the fail early approach and as a result limit expense.
Bringing all the necessary players together to create this platform will ensure that the use of cell therapies in solid tumor patients has a much higher chance of being a success where other treatment options have failed. In so doing, these patients may look forward to the same outcomes that cancer-free patients such as Emily Whitehead are now experiencing.
Financial & competing interests disclosure
The authors have no relevant financial involvement with an organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock options or ownership, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
References
1. Gross G, Esher Z. Therapeutic Potential of T Cell Chimeric Antigen Receptors (CARs) in Cancer Treatment: Counteracting Off-Tumor Toxicities for Safe CAR T Cell Therapy. Annu. Rev. Pharmacol. Toxicol. 2016; 56: 59–83. CrossRef
2. Di Stasi A, Tey SK, Dotti G et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011; 365(18): 1673–83. CrossRef
3. Grigor EJM, Fergusson DA, Haggar F et al. Efficacy and safety of chimeric antigen receptor T-cell (CAR-T) therapy in patients with haematological and solid malignancies: protocol for a systematic review and meta-analysis. BMJ Open 2017; 7(12): e019321. CrossRef
4. Santos EB, Yeh R, Lee J et al. Sensitive in vivo imaging of T cells using a membrane-bound Gaussia princeps luciferase. Nat Med. 2009; 15(3): 338-44. CrossRef
5. Kershaw MH, Westwood JA, Parker LL et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Can. Res. 2006; 12: 6106–15. CrossRef
6. Emami-Shahri N, Papa S. Dynamic imaging for CAR-T-cell therapy. Biochem. Soc. Trans. 2016; 44(2): 386–90). CrossRef
7. Dovedi SJ, Melis MH, Wilkinson RW et al. Systemic delivery of a TLR7 agonist in combination with radiation primes durable antitumor immune responses in mouse models of lymphoma. Nat. Med. 2009; 15(3): 388-44.
8. Lim WA, June CH. The Principles of Engineering Immune Cells to Treat Cancer. Cell 2017; 168(4):724–40. CrossRef
9. Melero I, Rouzaut A, Motz GT, Coukos G. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov. 2014; 4: 522–26. CrossRef
10. Srinivas M, Heerschap A, Ahrens ET et al. (19)F MRI for quantitative in vivo cell tracking. Trends Biotechnol. 2010; 28(7): 363–70. CrossRef
11. Lim WA, June CH. The Principles of Engineering Immune Cells to Treat Cancer. Cell 2017; 168(4): 724–40. CrossRef
12. Srinivas et al MRM 62(3):747-53 (2009); Aarntzen, Srinivas et al Proc Natl Acad Sci USA 108(45):18396-9 (2011).
13. Koshkina O, Lajoinie G, Bombelli FB et al. Multicore Liquid Perfluorocarbon‐Loaded Multimodal Nanoparticles for Stable Ultrasound and 19F MRI Applied to In Vivo Cell Tracking. Adv. Funct. Mat. 1806485 (2018) CrossRef
14. Srinivas M, Cruz LJ, Bonetto F et al. Customizable, multi-functional fluorocarbon nanoparticles for quantitative in vivo imaging using 19F MRI and optical imaging. Biomaterials 2010; 31(27): 7070–7. CrossRef
15. Adachi K, Kano Y, Nagai T et al. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 2018; 36: 346–51. CrossRef
16. Naumova AV, Modo M, Moore A et al. Clinical imaging in regenerative medicine. Nat. Biotech. 2014; 12(8) 804–18. CrossRef
Affiliations
D Morrow1, M Srinivas1,2, C Mann3, A Ussi1 & AL Andreu1
1 EATRIS ERIC, European Infrastructure for Translational Medicine, Amsterdam, Netherlands
2 Radboud University Medical Center (Radboud UMC), Department of Tumor Immunology, Radboud Institute for Molecular Life Sciences (RIMLS), Nijmegen, Netherlands
3 Asphalion S.L, Barcelona, Spain

This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License.