FectoVIR®-AAV: a giant step for AAV large scale manufacturing
Cell & Gene Therapy Insights 2020; 6(4), 655–661
10.18609/cgti.2020.077
The number of advanced therapy medicinal products (ATMPs) to treat inherited genetic disorders is in constant growth, with a global 32% increase in new clinical trials in the last 4 years. ATMPs have demonstrated their success with already more than ten approved for commercialization. The success of AAV as the most promising viral vector for gene therapy is due to low immunogenicity, broad tropism and non-integrating properties. One major challenge for translation of promising research to clinical development is the manufacture of sufficient quantities of AAV. Transient transfection of suspension cells is the most commonly used production platform, as it offers significant flexibility for cell and gene therapy development. However, this method shows some limitations in large scale bioreactors: inadequate transfection protocol, reduced transfection efficiency and lower productivity. To address this concern, we present data on the novel transfection reagent FectoVIR®-AAV specifically developed to bring flexibility of transient transfection together with scalability and speed to market.
Gene and gene-modified cell therapy treatments have demonstrated their potential in addressing unmet medical needs across a wide variety of human diseases, including cardiovascular, neurodegenerative, ocular, immunologic disorders and cancer. These therapies are based on the delivery of corrective DNA (DeoxyriboNucleic Acid) into target cells with viral vectors. These viral vectors can either be directly administered to patients, referred to as in vivo therapy, or first administered ex vivo to cells isolated from the patient (autologous therapy) or from a donor patient (allogenic therapy). Based on the Alliance for Regenerative Medicine’s 2019 annual Report, the number of approved gene therapy products has grown with the approval of two additional gene therapies for distinct indications (Zolgensma® for spinal muscular atrophy and Zynteglo® for beta thalassemia), and the number of gene therapies is expecting to double within the next 2 years [1]Alliance for Regenerative Medicine Q3 2019 Data Report: https://alliancerm.org/publication/q3-2019-data-report. The two viral vectors that are mostly used for gene and gene-modified cell therapy are recombinant adeno-associated virus (AAV) and lentivirus (LV). AAVs have particularly shown to be interesting because they are efficient for gene delivery to specific cell types, such as motor neurons (ex. Zolgensma®) and retinal cells (ex. Luxturna®).
Production of AAV viral vectors generally requires the co-transfection of three plasmids, with the first containing genes coding for the capsid proteins and necessary auxiliary genes, the second harboring the therapeutic gene of interest, and the third corresponding to the adenovirus-derived helper plasmid. These plasmids need to be delivered into mammalian cell lines, mostly human embryonic kidney 293 (HEK-293) cell lines and derivatives, which will be the host to synthesize recombinant AAV viral particles containing the therapeutic gene. The efficiency of the delivery process is essential to obtain a high number of producing cells. This delivery process is mainly dependent on the transfection method used. Transfection should ensure that plasmids are co-delivered into the highest number of cells and that the plasmids are protected from degradation before reaching the nucleus. In addition, the transfection method should be scalable and highly reproducible in order to support large scale viral vector manufacturing.
In a previous article [2]Nyamay’Antu A, Dumont M, Kedinger V, Erbacher P. Non-Viral Vector Mediated Gene Delivery: the Outsider to Watch Out For in Gene Therapy. Cell Gene Ther. Ins. 2018; 51–7., we discussed the advantages of using transient transfection for large scale viral vector manufacturing, which remains to this day the simplest and fastest approach to ensure production of viral particles. Of the existing transfection methods, the use of PEI-based transfection reagent is predominant in gene therapy as it combines affordability and compatibility for transfection of adherent and suspension cells. PEIpro® transfection reagent is a highly qualified PEI that has become the gold standard for large-scale production of viral vectors such as adenovirus, lentivirus and AAVs both in adherent and suspension systems [3]Gilbert R. Key considerations for the use of suspension culture systems for viral vector manufacturing. Cell Gene Ther. Ins. 2020; 6(1): 143–8., [4]Bauler M, Roberts JK, Wu CC et al. Production of Lentiviral Vectors Using Suspension Cells Grown in Serum-free Media. Mol. Ther. Methods. Clin. Dev. 2020; 17: 58–68., [5]Collaud F, Bortolussi G, Guianvarc’h L et al. Preclinical Development of an AAV8-hUGT1A1 Vector for the Treatment of Crigler-Najjar Syndrome. Mol. Ther. Methods Clin. Dev. 2018; 12: 157–74., [6]Heldt CL. Scalable method utilizing low pH for DNA removal in the harvest of recombinant adeno-associated virus vectors. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019; 1124: 173–9., [7]Cervera L, Kamen AA. Large-Scale Transient Transfection of Suspension Mammalian Cells for VLP Production. Methods Mol. Biol. 2018; 1674: 117–27., [8]Valkama AJ, Leinonen HM, Lipponen EM et al. Optimization of lentiviral vector production for scale-up in fixed-bed bioreactor. Gene Ther. 2018; 25: 39–46., [9]. The availability of this reagent at GMP (Good Manufacturing Practices) grade since 2018, has made PEIpro®-GMP the first transfection reagent that is compliant with international GMP guidelines and is suitable for ATMPs manufacturing and commercialization.
Current needs for large scale AAV manufacturing
The new challenge is now to develop production platforms that are capable of answering the growing needs for commercial manufacturing. Current conventional methods for virus production are based on adherent cell culture systems in the presence of serum, which are suboptimal set-ups when the aim is to put in place a validated manufacturing process to produce at large scale reproducible viral yields. Based on the manufacturing process adopted for protein and antibody manufacturing, the growing tendency among AAV viral vector manufacturers is to move on to suspension producer cells grown in chemically defined synthetic medium. By switching to suspension cell culture systems, the aim is 3-fold: reduce batch-to-batch variability by eliminating fluctuating parameters of cell culture (e.g. serum, cell seeding density), simplify downstream harvesting and purification processes, and increase AAV production to treat larger group of patients.
Because the gene and therapy field is progressing at a fast pace, so should technologies to support the feasibility of producing more at larger scale without compromising on quality. Large scale bioreactors for the culture of suspension cells are growingly used and this increase in scale can lead to lower yield that can be explained by: i) physical and mechanical constraints that impact cellular metabolism in large scale cell culture; and ii) time and volume constraints with the handling of bigger transfection volumes. With 20 years of expertise in transfection, Polyplus-transfection has tackled large scale transfection constraints by developing a novel transfection reagent. FectoVIR®-AAV is a novel class of animal free transfection reagent specifically developed for large scale transfection to improve scalability, productivity and flexibility for industrial manufacturing of AAV viral vectors in suspension cells.
Improving large scale transfection: production yield
Transfection whether at small or large scale is dependent on the efficiency of the delivery molecule. As such, it is essential to identify a delivery molecule that is optimal for a given application. For AAV viral vector production in suspension cells, we screened a refined chemical library with specific criteria: scalability and improved production yields. These additional physico-chemical specifications were also essential to retain FectoVIR®-AAV as lead candidate: animal-free, scalable synthesis and GMP-grade compatibility. In comparison to the gold standard PEIpro® and other competitors used for viral vector manufacturing, FectoVIR®-AAV improved significantly rAAV2 production yield in suspension cells with up to 10-fold increase in functional titer yields (Figure 1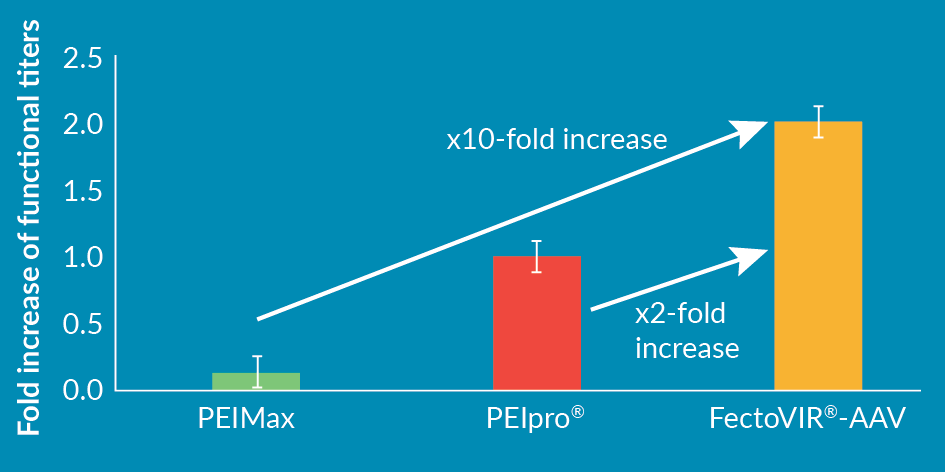
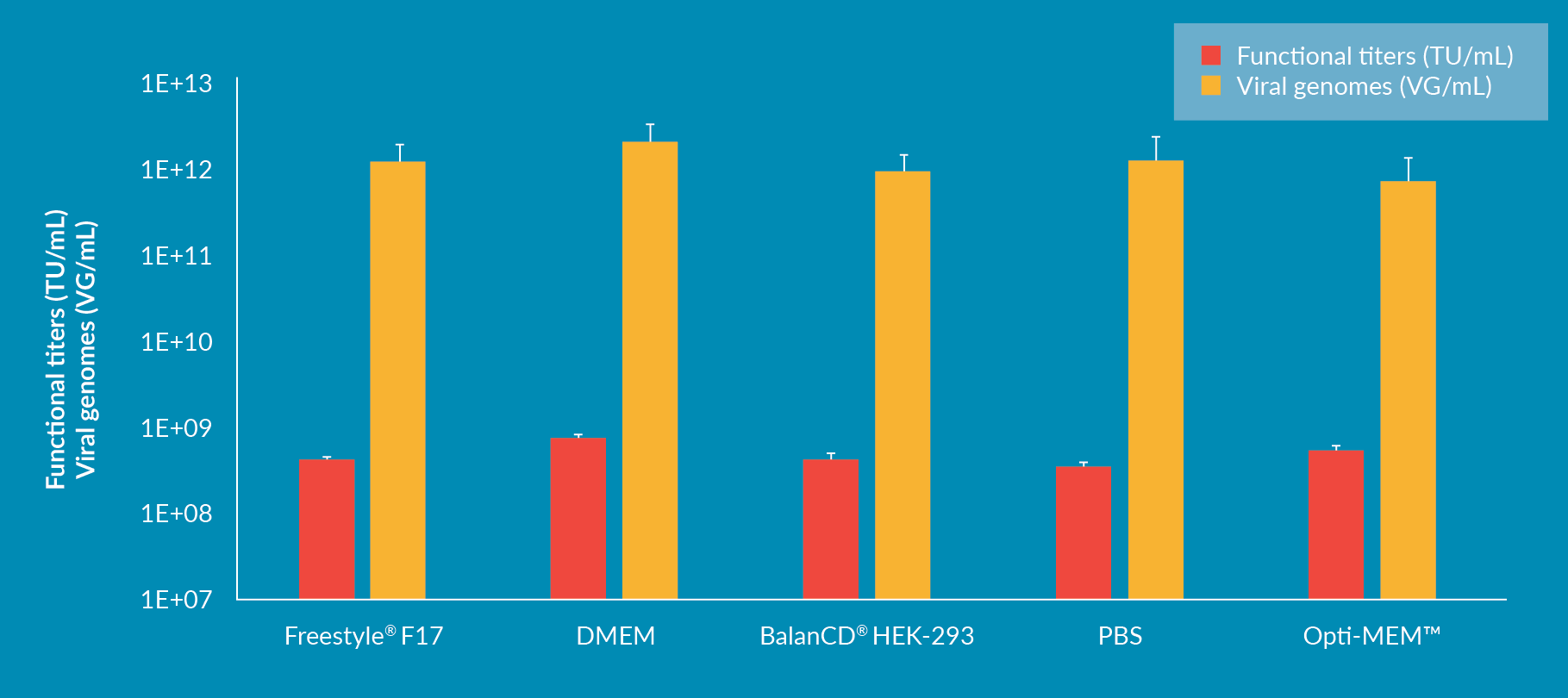
Improving large scale transfection: scalability
Transfection at small scale requires the pre-mixing of DNA and transfection reagent to form complexes that are incubated at room temperature for a given amount of time, usually within a short 15–30-minute window to prevent their aggregation and allow efficient binding to the cell membrane and subsequent endocytosis. Once formed, these transfection complexes are immediately added to the cell culture. The pre-mixing volume usually represents 10% of the cell culture volume, and at small scale it is manageable to quickly add this volume. When moving on to large scale transfection, it consequently leads to working with large volumes during preparation of transfection complexes and during their transfer into the bioreactor. The implementation of a transfection protocol compatible with upscaling is therefore indispensable to maintain high viral titer yields. FectoVIR®-AAV transfection reagent addresses both time and volume constraints of large-scale transfection. As shown in Figure 3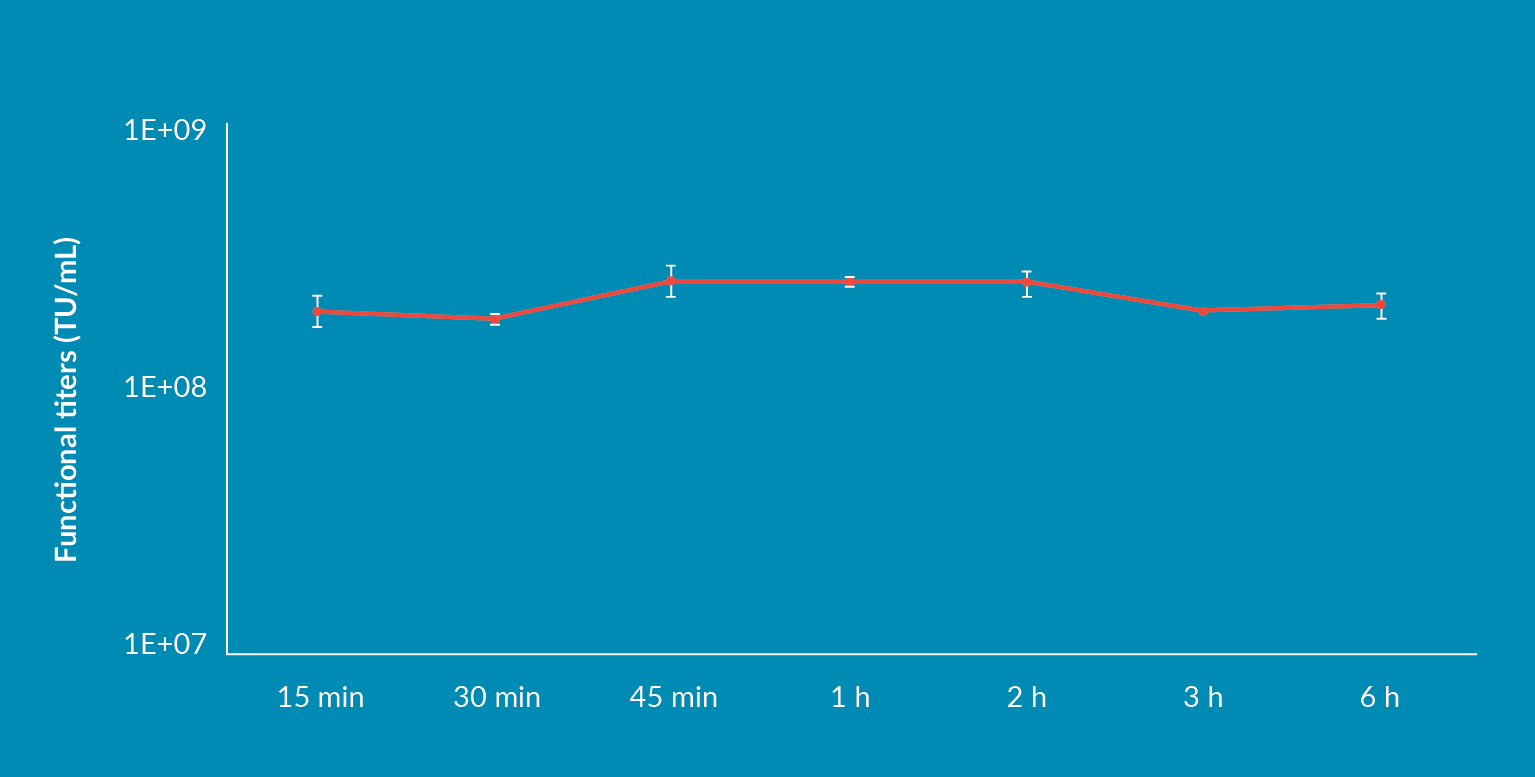
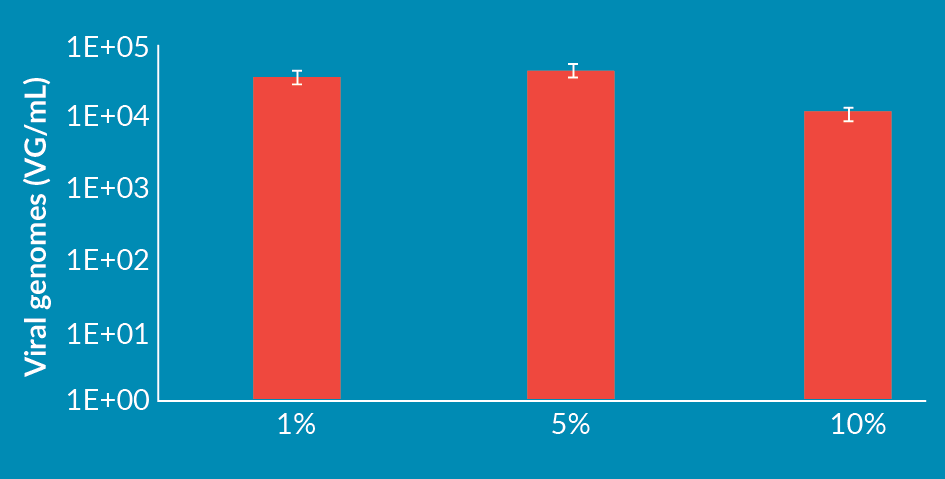
Short-term objective: meeting quality requirements
With the growing AAV manufacturing capacity demands, the need for novel technologies to aid viral manufacturers increase production is critical. It is also indispensable that all raw materials used for the production of therapeutic viral vectors meet quality requirements for future commercialization. Polyplus-transfection® has acquired a strong expertise in developing GMP grade transfection reagent, also recently marked by the launch of the first GMP compliant transfection reagent for therapeutic viral vector manufacturing (PEIpro®-GMP in 2018). The strategy for developing GMP-grade transfection reagent is a fully validated process in compliance with GMP guidelines to ensure traceability from starting material to the final product. Therefore, during initial identification of lead candidate FectoVIR-AAV®, we confirmed its GMP compatibility and the feasibility of its synthesis at large scale which now allows us to confirm that it will be commercially available starting Q2 2021, concomitantly with a residual test to ensure FectoVIR-AAV’s traceability throughout AAV manufacturing process.
References
1. Alliance for Regenerative Medicine Q3 2019 Data Report: https://alliancerm.org/publication/q3-2019-data-report Crossref
2. Nyamay’Antu A, Dumont M, Kedinger V, Erbacher P. Non-Viral Vector Mediated Gene Delivery: the Outsider to Watch Out For in Gene Therapy. Cell Gene Ther. Ins. 2018; 51–7. Crossref
3. Gilbert R. Key considerations for the use of suspension culture systems for viral vector manufacturing. Cell Gene Ther. Ins. 2020; 6(1): 143–8. Crossref
4. Bauler M, Roberts JK, Wu CC et al. Production of Lentiviral Vectors Using Suspension Cells Grown in Serum-free Media. Mol. Ther. Methods. Clin. Dev. 2020; 17: 58–68. Crossref
5. Collaud F, Bortolussi G, Guianvarc’h L et al. Preclinical Development of an AAV8-hUGT1A1 Vector for the Treatment of Crigler-Najjar Syndrome. Mol. Ther. Methods Clin. Dev. 2018; 12: 157–74. Crossref
6. Heldt CL. Scalable method utilizing low pH for DNA removal in the harvest of recombinant adeno-associated virus vectors. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019; 1124: 173–9. Crossref
7. Cervera L, Kamen AA. Large-Scale Transient Transfection of Suspension Mammalian Cells for VLP Production. Methods Mol. Biol. 2018; 1674: 117–27. Crossref
8. Valkama AJ, Leinonen HM, Lipponen EM et al. Optimization of lentiviral vector production for scale-up in fixed-bed bioreactor. Gene Ther. 2018; 25: 39–46. Crossref
Affiliations
Alengo Nyamay’antu, PhD
Scientific Communication Specialist, Polyplus-transfection® SA
Malik Hellal, PhD
Senior Scientist in Chemistry,
Polyplus-transfection® SA
Mathieu Porte, PhD
R&D Manager Bioproduction,
Polyplus-transfection® SA
Patrick Erbacher, PhD
CSO, Polyplus-transfection® SA
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: The authors are employees of Polyplus Transfection. They declare that they have no conflicts of interest.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2020 Nyamay’antu A, Hellal M, Porte M & Erbacher P. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Apr 16 2020; Revised manuscript received: May 18 2020; Publication date: May 28 2020.

