Novel equipment and process changes: implications for your manufacturing strategy
Cell & Gene Therapy Insights 2019; 5(5), 819-828.
10.18609/cgti.2019.090
With Cell and Gene Therapies transitioning through clinical trials and into commercial approvals at an unprecedented speed, process optimization steps are often considered late in the process. Alongside this fast-moving clinical development, tools providers are working hard to optimize equipment to better serve these new modalities. Evaluation, and implementation, of new or novel equipment is therefore, an ongoing process across the industry. Once INDs (or CTAs) have been filed, and first patients have been treated, onboarding new equipment constitutes a major manufacturing change. Such changes need to be appropriately communicated to the regulatory bodies, and thus managed from a data submission and risk profile perspective. In this article, we outline the main considerations from a broad GMP equipment compliance perspective, as well as indicating key resources and referencing guidelines for both Europe and the United States on how to navigate the regulatory aspects of manufacturing changes.
We recently discussed the need for tailor-made equipment to suitably serve the cell and gene therapy (CGT) industry and its growing manufacturing needs [1]. At the core of this statement lies the need for high quality cells, in sufficient doses, in order to provide seriously sick patients with the best possible treatments.
Current manufacturing approaches, both during clinical trial stages, and in some of the recently approved commercial treatments, tend to still be labor intensive [2]. They are based on a range of technologies typically found in a discovery laboratory environment, paired with technologies that have been proven useful in biologics manufacturing and blood processing. This approach has worked for small patient numbers, where manufacturing capacity and experienced manufacturing personnel were not limiting factors. Improving manufacturing processes has, however, become a recurring theme now that the field is seeing indication expansions, and more importantly applicability of CGT treatments in larger indications, thus mandating significantly higher numbers of doses.
One fundamental aspect to solving some of the manufacturing issues is a better understanding of the product in general, and, more specifically, understanding the critical quality attributes. Novartis has been reported to address this challenge with an Industry 4.0 approach, banking on data and Artificial Intelligence [3]. Once a product is better understood, so the thinking goes, there is a potential that smaller but more potent doses will be just as effective (if not more so) as the current approaches; think University of Pennsylvania’s finding that 94% of Emily Whitehead’s treatment success can be traced back to one single clone [4].
In addition to reducing the required cell numbers as one approach to countering manufacturing constraints, there is broad consensus that having suitable tools and equipment to further enable the manufacture, of what are after all living organisms, will be key. With a range of new ‘made for purpose’ tools entering the CGT space, we routinely encounter the question of the ideal timepoint to implement new equipment to improve manufacturing outcomes. In this article, we discuss the different considerations when introducing novel manufacturing technologies, with a focus on commercial and regulatory implications.
Theory vs reality
It is widely recommended to start with the end goal in mind, i.e., what would a process need to look like in order to supply a commercial market with the predicted patient numbers? To put it more broadly, when designing a manufacturing strategy, it is critical to consider quality, cost of goods, distribution, sustainability and scalability right from the start. With the right strategy and long-term view, costly and often difficult and time-consuming manufacturing changes can be avoided at a later stage. In an ideal world, this would mean integrating the appropriate manufacturing technologies at the preclinical stage, optimizing them through the early clinical phase, and locking down a process for the pivotal trial – the so-called Development by Design approach [5] and similar concepts.
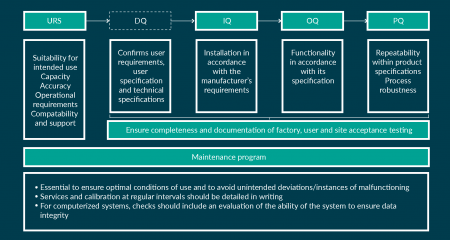
Figure 1: Outline of qualification activities and maintenance program requirements to for GMP compliant
manufacturing setups.
While this strategic approach is broadly accepted and makes rational sense, the reality is impacted by a range of external factors: Typically, CGT developers are small to medium sized companies in a pre-revenue situation. While being small allows them to be nimble and flexible, their funding milestones tend to be based on clinical achievements such as Clinical Trial Application (CTA)/Investigational New Drug (IND) application filed, or first patient treated, etc. This inevitably means that timelines need to be weighed up against process improvements, more often than not with the outcome that relatively manual processes will enter the clinic and are even carried through to a pivotal trial. In other words, and to quote an industry colleague: “Science usually trumps process.” It is not uncommon to introduce more appropriate technologies late in development or even post-approval.
The drivers that ultimately trigger such a process change tend to vary from company to company but can broadly be summarized in three categories: manufacturing robustness, scalability and cost. (i) Manufacturing robustness will be the main consideration for manual processes: they tend to be marred by operator variability, as well as the risk of contamination due to open manipulation steps. Where available, automation through suitable equipment can be a remedy, and therapy developers will always be on the lookout for novel equipment that could provide a solution to close a process. (ii) Scalability will be the driver for medium to large indications. While early phase trials tend to address limited patient numbers, most modalities will ultimately experience an indication expansion to increase the return on development investment. This will lead to an increase in dose numbers, and manufacturing processes that were initially designed for a smaller addressable market may no longer be suitable. Equipment can be one approach to enable both scale-up and scale-out. (iii) Finally, in an industry where treatment costs can reach the million-dollar mark, the cost of goods has been under tight scrutiny [6]. Investigating the individual cost drivers would be another article in itself; however, it is safe to say that novel equipment is expected to play an integral part in reducing cost, by enabling a reduction in hands-on time (labor cost), more efficient use of space by combining and automating unit operations (facility cost), automating testing (quality assurance cost) and reducing the regulatory burden by lowering the facility classification requirements through novel closed and fully automated equipment (monitoring cost), to name but a few [7].
Depending on the developmental stage, different regulatory aspects need to be considered. When equipment is introduced during the pre-clinical stage, developers have a broad portfolio of technologies to choose from. Once an IND filing for the USA, or an CTA filing for the EU, is prepared, the contents for the Chemistry, Manufacturing and Controls (CMC) section start to get populated: the initial focus during the early clinical phases will be on the manufacturing process itself. While manufacturing technologies are referenced, their full documentation, and suitability for larger scale manufacturing, will not be tightly scrutinized until a pivotal phase with line of sight for commercialization is initiated. To avoid unexpected pushback due to unsuitable equipment or missing documentation, we have outlined some of the quality and regulatory considerations for equipment selection. While they are not intended to be exhaustive and will not replace direct conversations with regulators, we hope to contribute to a broader understanding of the interplay of manufacturing processes, equipment and regulations.
Equipment considerations & good manufacturing practice
Equipment is intrinsic to and a key consideration when manufacturing CGT products. Its understanding is as important as the nature of the product itself, and the numerous variables that factor into the manufacturing process. The impact of changing equipment once a drug product is authorized by regulatory agencies for clinical use needs to therefore be considered carefully: any change to the equipment will require qualification to confirm the equipment is fit for purpose and does not impact the process or the product quality.
When choosing new equipment, it should be of a suitable size and construction to facilitate cleaning, maintenance and proper process operations for any given therapeutic product (see our GMP checklist for equipment Box 1). The ability for adequate cleaning and disinfection of the equipment is required to ensure aseptic conditions for processing. Fail safe modes should be built into automated equipment design and associated computer systems to ensure no compromise to the process/product. Clearly defined User Requirement Specification (URS), potentially a Design Qualification (DQ), as well as detailed Installation, Operational, Performance Qualification (IQ/OQ/PQ), relevant acceptance testing, and maintenance procedures are further prerequisites (see Figure 1 for further explanations). The European Commission has established detailed guidelines for GMP, Eudralex Volume 4, in particular Chapter 3, and similar regulations, such as 21 CFR Subpart D (Equipment), are in effect in the USA, established by the Food and Drug Administration (FDA). Tables 1 & 2 detail regulatory frameworks relevant to equipment currently in effect in Europe and the USA, for further guidance.
| Table 1: Relevant GMP guidelines | |
|---|---|
| European Union | |
| Two key legal instruments on the principles and guidelines of cGMP for medicines, specifically for active substances and medicines for human use | Regulation No. 1252/2014 [17] |
| Directive 2003/94/EC [18] | |
| Overall interpretation of these guidelines including a rich annex with further details and examples | EU cGMP guidelines [19] |
| GMP considerations for ATMPs, specifically | Regulation (EC) No 1394/2007 [21] |
| GMP Guideline for ATMPs [22] | |
| United States | |
| Guidance for all pharmaceutical products | 21 CFR Part 211 [24] |
| Guidance for biological products | 21 CFR Part 600 [25] |
| Guidance for cell and tissue-based products, specifically | 21 CFR Part 1271 [23] |
| Global | |
| Good manufacturing practice guide for active substance manufacture, recommended for adoption in the EU, USA, and Japan | ICH Q7 [20] |
Even if equipment and the intended manufacturing context is considered appropriate from a regulatory standpoint, and all relevant documentation to underpin this has been compiled, it needs to be validated in the context of the specific manufacturing process. Elements of validation apply as soon as a Phase 1 is initiated; requirements for validation will increase as the product nears the market and will need to be appropriately documented if novel equipment is introduced. Qualification should take into account all critical factors, for example equipment sanitization and the integrity of the equipment. Equipment should be re-evaluated at appropriate intervals to confirm that it remains suitable for the intended operations. When using computerized systems, their validation should be proportionate to the impact on the quality of the product; consideration should be given to GAMP 5 [8], EU GMP Vol 4 Annex 11 [9] and to the FDA guidance 21 CFR Part 11 [10].
| Box 1: GMP Equipment Check List |
|---|
| ✔ The equipment is designed, located and maintained to suit its intended purpose. |
| ✔ Repair and maintenance operations do not present any hazard to the quality of the products. |
| ✔ The equipment is designed so that it can be easily and thoroughly cleaned. It should be cleaned according to detailed and written procedures and stored only in a clean and dry condition. |
| ✔ Washing and cleaning equipment is chosen and used in order not to be a source of contamination. |
| ✔ Equipment is installed in such a way as to prevent any risk of error or of contamination. |
| ✔ The equipment does not present any hazard to products. Parts of equipment that come into contact with the product must not be reactive, additive or absorptive to such an extent that it will affect the quality of the product and thus present any hazard. In addition, parts of the equipment that come into contact with cells/tissues should be sterile and of appropriate quality for the purpose. |
| ✔ Balances and measuring equipment of an appropriate range and precision be available for production and control operations. |
| ✔ Measuring, weighing, recording and control equipment be calibrated and checked at defined intervals by appropriate methods. Adequate records of such tests should be maintained. |
| ✔ Fixed pipework is clearly labelled to indicate the contents and, where applicable, the direction of flow. |
| ✔ Defective equipment, if possible, be removed or at least be clearly labelled as defective. |
Lastly, equipment cleaning procedures and cleaning reagents should be chosen carefully. In most cases, equipment providers will recommend tried and tested approaches; equipment materials and design will ideally have been chosen in a manner that is safe and appropriate for GMP implementation. To simplify changeover and cleaning procedures between runs, most providers in the CGT space have taken lessons from the bioprocess industry and apply a single-use concept, thus minimizing the risk of (cross-) contaminations [11]. In comparison to stainless steel containers with their cleaning requirements, single-use consumables come with their own set of considerations: made from various types of plastic or polymer films, sterilization technologies such as gamma irradiation need to be implemented and validated. Extractables and leachables data sets should provide proof that the chosen material is fit for use – this is particularly important for material that comes into contact with cells, media and buffers [12]. And last, but not least, single-use means exactly that: these production consumables will have to be disposed off after one use, which not only generates questions around the environmental impact, but also concerns over potentially hazardous material left in bags, tubing or other containers.
Data considerations for process improvements
Assuming the equipment satisfies GMP requirements and meets all other regulatory standards, a change in equipment once clinical studies have been initiated may signify a ‘substantial’ manufacturing change. At the very least, comparability runs will need to be performed, which should demonstrate that the resulting product conforms to the same quality specifications, and to demonstrate equivalence of batches. If the outcome of these runs shows differences, the existing product knowledge should be sufficiently predictive to ensure that this has no adverse impact on the safety or efficacy of the therapeutic, as per guidance ICH Q5E [13].
The extent of the comparability requirements during the clinical development phase depends on the specific stage of development, the availability of analytical procedures, and the extent of product and process knowledge. Consequently, comparability testing during early development (up to Phase 1/2) tends to be less extensive than for an approved product, with the focus being on safety. It should be noted that, if changes are introduced in late development, and no additional clinical studies are planned to support a Biologics License Application (BLA) in the USA, or a Marketing Authorisation Application (MAA) in the EU, the comparability requirements could be as comprehensive as for an approved product. For interested readers, Dr Joslyn Brunelle from the FDA presented a range of case studies in a talk titled “FDA recommendations for comparability studies to support manufacturing changes” [14]; while the modalities considered are mostly small molecules and biologics, it provides a good overview on the increase in data requirements in relation to the clinical development stage.
| Box 2: Case Study |
|---|
| FloDesign Sonics customer Alpha*: |
| ▪ Mid-sized US start-up company, venture-funded in a series B, working towards finishing their clinical phase 1/2a milestone in the US, which will trigger the next funding round and a move into phase 3/pivotal with the aim of applying for a US BLA. |
| Product and current process*: |
| ▪ Allogeneic MSCs grown on microcarriers, currently in a 3 L stir tank bioreactor, with the long-term aim of expanding to up to 200L. To scale, the open, semi-manual manipulations in the biosafety cabinet and using a centrifuge are going to be replaced with the novel ekko™ platform. The proposed changes are also expected to improve yield and some quality aspects. |
| Proposed process changes: |
| ▪ Introduction of a novel equipment (ekko™), which will close the process, and fully automate the major unit operations outside of the bioreactor. |
| Resulting product changes: |
| ▪ Demonstrated increased uniformity of MSC coverage on microcarriers during culture, still within IND-listed range. |
| ▪ Demonstrated increased viability due to removal of non-attached single cells, still within IND-listed range. |
| ▪ Demonstrated increased efficiency in microcarrier residual removal, well below IND-listed range. |
| ▪ Demonstrated change in surface marker expression, while no morphological changes were noted. |
| Regulatory steps to consider: |
| 1. File an investigational IND quality information amendment, containing: |
| ▪ Details of process changes |
| ▪ GMP-relevant documentation and data sets for novel equipment (unless Master File reference is available and contains all relevant data sets) |
| ▪ Timelines of process change implementation |
| ▪ Comparability data |
| ▪ Existing product knowledge details confirming continued product quality despite changes |
| 2. Request Type C meeting to discuss severity of surface marker changes. |
| 3. Expect additional toxicity data requirements, and potential potency data to confirm equivalence of the new quality profile. |
| 4. Potential need to add more patients to the phase 1/2a to demonstrate efficacy. |
| *Fictitious customer and process, any and all similarities are coincidental. |
Overall, it is recommended to implement any manufacturing process and/or equipment changes during early phases of clinical studies (i.e., prior to initiating a Phase 3/pivotal study). These changes should be communicated to the FDA through an investigational IND quality information amendment, or in the EU as an amendment to the clinical trial authorization application to the appropriate member state. The extent of the changes should be clearly outlined, with a projected timeline as to when these changes will be introduced into clinical manufacturing. In the USA, a Type C meeting might be warranted to discuss the comparability exercise; for the EU, advice can be sought through the Scientific Advice procedure with a regulatory or scientific advice meeting.
Should changes post BLA or MAA be warranted, these changes need to be reported according to regulatory requirements. A draft guidance summarizing these regulations has been issued by the FDA at the end of 2017 [15] and is currently being finalized. In the EU, any change to the MA will be through Variation submission [16]. Please also see Tables 1 & 2, which references the most relevant regulations.
| Table 2: Relevant Guidelines and Recommendations for Equipment Changes post-Approval. | |
|---|---|
| European Union | |
| ICH Q5E: Comparability of Biotechnological/Biological Products Subject to Changes in their Manufacturing Process [12] | |
| Note for Guidance on Biotechnology/Biological Products Subject to Changes in Their Manufacturing Process [29] | |
| EMA Questions and Answers on Gene Therapy [30] | |
| EMA Guideline on the Quality, Non-Clinical and Clinical Aspects of Gene Therapy Medicinal Products [31] | |
| United States | |
| Reporting of equipment changes post-BLA Changes need to comply with a range of regulations | 21 CFR 601.12 [33] |
| |
| Requirements for making and reporting manufacturing changes to an approved BLA, and for distributing a licensed product made with such a change | Section 506A of the FD&C Act (21 U.S.C. 356a) [35] |
| For further context |
|
| Global | |
| Recommended Reading: ISPE Good Practice Guide, Applied Risk Management for Commissioning and Qualification [32] | |
In Summary
A clear focus when moving a cell or gene therapy through clinical development should be on designing the process with manufacturability and commercial market supply in mind. This includes raw material selection, supply chain and logistics considerations, and the appropriate manufacturing equipment to ensure the best possible drug product quality. Any changes introduced after a product has already been used in humans tend to be costly and time consuming. However, despite all the outlined requirements and considerations that come with manufacturing and/or process changes, process improvements should generally be encouraged. Changes that can be shown to: (i) enable reliable manufacturing; (ii) underpin overall supply assurance; and/or (iii) improve the quality profile of a given CGT product, may be perceived as favorable and have seen a good level of support from regulatory agencies.
From an equipment supplier perspective, we encourage our customers to have an open dialogue about clinical timelines. This allows both sides to ensure that all GMP-related documentation can be assembled as needed, and comparability studies can be properly supported.
Acknowledgements
The author would like to thank the Regulatory Team at the Cell and Gene Therapy Catapult for their contributions and fact checking. Particular thanks go to Dr Zain Moola (Senior Manager, Regulatory Affairs) and Dr Jacqueline Barry (Chief Clinical Officer).
Financial & competing interests disclosure
NB is an employee of FloDesign Sonics but otherwise has no relevant financial involvement with an organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this manuscript. No writing assistance was utilized in the production of this manuscript.
References
1. Bauer N, Grant R. Moving from Retrofitting to Made for Purpose Manufacturing Equipment, Critical role of automation in the manufacture of cell and gene therapies. Cell and Gene Therapy Insights 2018; 4(9): 941–6. CrossRef
2. Novartis, still struggling with Kymriah manufacturing, is providing some out-of-spec doses to patients who ask; Eric Palmer; FiercePharma; December 18, 2018.
3. How to Solve a Problem like Kymriah?; Ben Hargraves; BioPharma Reporter; February 22, 2019.
4. Fraietta JA, Nobles CL, Sammons MA et al. Disruption of TET2 Promotes the Therapeutic Efficacy of CD19-targeted T-cells. Nature 2018; 558(7709): 307-12. CrossRef
5. Hampson B, Smith D. Development by design methodology: the key to successful manufacturing of patient-specific cell therapies. Drug Target Review July 11 2017.
6. Zolgensma: A Remarkable New Treatment, An ICER Analysis, And A Poorly Justified Price, Peter B. Bach, Health Affairs Blog, June 18, 2019: Website
7. Novel Equipment Solutions: Leading to Manufacturing Efficiency and Productivity, Guy Tiene, Pharma’s Almanach, August 1 2016: Website
8. ISPE GAMP® 5 Guide: A Risk-Based Approach to Compliant GxP Computerized Systems: Website
9. EudraLex The Rules Governing Medicinal Products in the European Union Volume 4 Good Manufacturing Practice Medicinal Products for Human and Veterinary Use Annex 11: Computerised Systems: Website
10. Guidance for Industry Part 11, Electronic Records; Electronic Signatures – Scope and Application: Website
11. Cell & Gene Therapies: A Guide to Single-Use Connections – 10 Transferable Lessons from the Bioprocessing Industry; Derek Pendlebury; CPCWorldwide.com, white paper, 2018.
12. Single-use systems for biotechnology products, Scott Rudge, European Pharmaceutical Review, February 12, 2018: Website
13. Q5E Comparability of Biotechnological/Biological Products Subject to Changes in Their Manufacturing Process: Website
14. FDA recommendations for comparability studies to support manufacturing changes; Joslyn Brunelle: Website
15. Chemistry, Manufacturing, and Controls Changes to an Approved Application: Certain Biological Products; Draft Guidance for Industry: Website
16. European Commission Variations Guideline: Website
17. Commission Delegated Regulation (EU) No 1252/2014 of 28 May 2014 supplementing Directive 2001/83/EC of the European Parliament and of the Council with regard to principles and guidelines of good manufacturing practice for active substances for medicinal products for human use Text with EEA relevance: Website
18. COMMISSION DIRECTIVE 2003/94/EC of 8 October 2003 laying down the principles and guidelines of good manufacturing practice in respect of medicinal products for human use and investigational medicinal products for human use: Website
19. EudraLex – Volume 4 – Good Manufacturing Practice (GMP) guidelines: Website
20. ICH harmonised tripartite guideline Good Manufacturing Practice guide for active pharmaceutical ingredients Q7: Website
21. Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004: Website
22. EudraLex The Rules Governing Medicinal Products in the European Union Volume 4 Good Manufacturing Practice Guidelines on Good Manufacturing Practice specific to Advanced Therapy Medicinal Products: Website
23. CFR21 Part 1271 – Human Cells, Tissues, and Cellular and Tissue-based Products: Website
24. CFR21 Part 211 – Current Good Manufacturing Practice for Finished Pharmaceuticals: Website
25. CFR21 Part 600 – Biological Products: General: Website
26. Changes to an Approved Application; Final rule (62 FR 39890, July 24, 1997): Website
27. Supplements and Other Changes to an Approved Application; Final rule (69 FR 18728, April 8, 2004).
28. Guidance for Industry: Changes to an Approved Application for Specified Biotechnology and Specified Synthetic Biological Products, July 1997. Website
29. ICH Topic Q 5 E Comparability of Biotechnological/Biological Products: Website
30. Committee for Medicinal Products for Human Use (CHMP) – Questions and Answers on Gene Therapy: Website
31. Guideline on the quality, non-clinical and clinical aspects 5 of gene therapy medicinal products (draft): Website
32. ISPE Good Practice Guide: Applied Risk Management in Commissioning and Qualification Website
Affiliation
Nina G Bauer
Chief Commercial Officer
FloDesign Sonics Inc., 380 Main Street, Wilbraham, MA 01095, USA
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License.