Improving the quality cell yield of T-cell immunotherapies through selective pressures imparted by culture media supplements
Cell & Gene Therapy Insights 2020; 6(2), 287–294
10.18609/cgti.2020.039
New T-cell based therapies use the adaptive immune system as a modality in multiple blood cancer indications and are being investigated in some solid tumor indications. This study looks at both total yield and memory character as measures of cell quality and those traits were used to evaluate human AB serum (ABS) and human platelet lysate (nLiven) as culture media supplements. Two independent labs showed statistically significant increases in both total cell yield and final T-cell central memory phenotype after expanding isolated cells in medium supplemented with nLiven as opposed to ABS. There was an additional, unexpected observation of increased donor to donor consistency when cultured with nLiven which may be a result of a more homogenous source of proteins and chemicals typically required to expand T-cells. Developing commercially viable manufacturing processes for T-cell-based therapies requires the adoption of new technologies that will facilitate process robustness. This study investigates media supplements within this context.
Engineered T-cell therapies, CAR-T and TCR, have emerged as a highly effective new therapeutic modality in blood cancers and are showing promise in solid tumor indications in the clinic. These treatments work by co-opting the immune system’s natural cancer fighting ability and targeting a surface antigen specific to the cancerous cell population. Utilizing a complicated biological system provides therapy developers with a powerful tool to direct against various diseases, however it also limits researchers’ understanding of the attributes that indicate the drugs’ efficacy. Historically, treatment doses are calculated based on the total number of cells presenting the surface antigen of interest with the hope that a portion of those cells would engraft in the patient and exhibit a persistent response. Recent research, however, has indicated that T cells that present a central memory phenotype (TCM) have increased efficacy over effector phenotypes across multiple disease models [1]Klebanoff CA, Gattinoni L, Torabi-Parizi P et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. PNAS 2005; 102 (27): 9571–6., [2]Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. J. Exp. Med. 2005; 202(1): 123–33., [3]Hislop AD, Gudgeon NH, Callan MFC et al. EBV-Specific CD8+ T Cell Memory: Relationships Between Epitope Specificity, Cell Phenotype, and Immediate Effector Function. J. Immunol. 2001; 167(4): 2019–29., [4]Jackola DR, Ruger JK. Miller RA. Age-associated changes in human T cell phenotype and function. Aging Clin. Exp. Res. 1994; 6: 25–3 .
Using cell memory character as a lever to increase therapeutic efficacy could have significant downstream effects for manufacturers and patients. If the treatment is more effective on a per cell basis, patients could be treated with a smaller minimum therapeutic dose. Manufacturers could have shorter manufacturing lengths, fewer materials, and increased process consistency, while patients could see a cheaper therapy that has a more consistent efficacy.
Another concern raised by therapeutics developers who have received or are anticipating market approval is the sustainability of their chosen media supplements. Historically, human primary cell culture has been limited to a small group of investigators, but recently the space has grown rapidly. That growth has put stress on the established supply chain. Fetal bovine serum has significant regulatory concerns as an animal derived reagent, so researchers have leaned heavily on human AB serum as a source of a particular mix of proteins, hormones, and cytokines that promotes growth in T-cell manufacturing processes. However, members of the industry are acutely concerned about the long-term availability of human AB serum which has to be sourced from voluntary, male donors of the AB serotype. The source of this material is relatively inflexible as only approximately 3% of the total population have the AB serotype, it cannot be scaled to meet demand like other reagents, and AB serum is used in the culture medium of most T-cell therapies [5]“Blood Types.” NHS Blood Donation: www.blood.co.uk/why-give-blood/blood-types/. The need for an alternative xeno-free supplement is rapidly approaching.
To investigate methods for improving cell quality and shore up supply concerns, a group at the Baylor College of Medicine, led by Norihiro Watanabe, performed a small-scale evaluation of the impact of various protein sources in culture media on T-cell memory phenotype and therapeutic efficacy using fetal bovine serum (FBS), human AB serum (ABS), and a uniquely processed human platelet lysate (nLiven PRTM). Their results show a statistically significant increase in T cells exhibiting central memory phenotypes and in total survival using a mouse model when cultured in nLiven PRTM versus ABS and FBS [6]Torres Chavez A, McKenna MK, Canestrari E et al. Expanding CAR T cells in human platelet lysate renders T cells with in vivo longevity. J. ImmunoTher. Cancer 2019; 7: 330.. Dr Watanabe’s team evaluated the in vivo efficacy of T-cell cultured in nLiven PRTM against both solid tumors and blood cancers. The study shows that the T cells expanded ex vivo with nLiven PRTM had statistically significant increases in the duration that cells were present in peripheral blood, total cells that were actively circulating, and the percent survival of the mice when compared to the same population of T cells expanded with either FBS or ABS. To further evaluate the impact of each medium supplement for therapeutic effect, the study evaluates engraftment by rechallenging the solid tumor model 21 days after the initial infusion. Again, the response by the cells expanded with nLiven PRTM was significantly more pronounced than in the cell populations expanded in either FBS or ABS.
Hitachi Advanced Therapeutics Solutions (HCATS) and Sexton Biotechnologies partnered to investigate if the human platelet lysate supplement nLiven PRTM would maintain these strong advantages when evaluated in more clinically representative culture models.
The first consideration for the experiments performed at Sexton and HCATS was to confirm that medium supplemented with nLiven PRTM produced a similar yield of total T cells to that of standard medium supplemented with ABS. At Sexton, peripheral blood mononuclear cells (PBMCs) were obtained from two donors (StemCell Technologies), activated with ImmunoCultTM Human CD3/CD28 T Cell Activator (StemCell Technologies), and cultured for fourteen days in static conditions. There was no significant change in cell expansion through the first 8 days of culture, however after day 10 the cultures using nLiven PRTM showed significantly higher expansion compared to ABS in both donors (Figure 1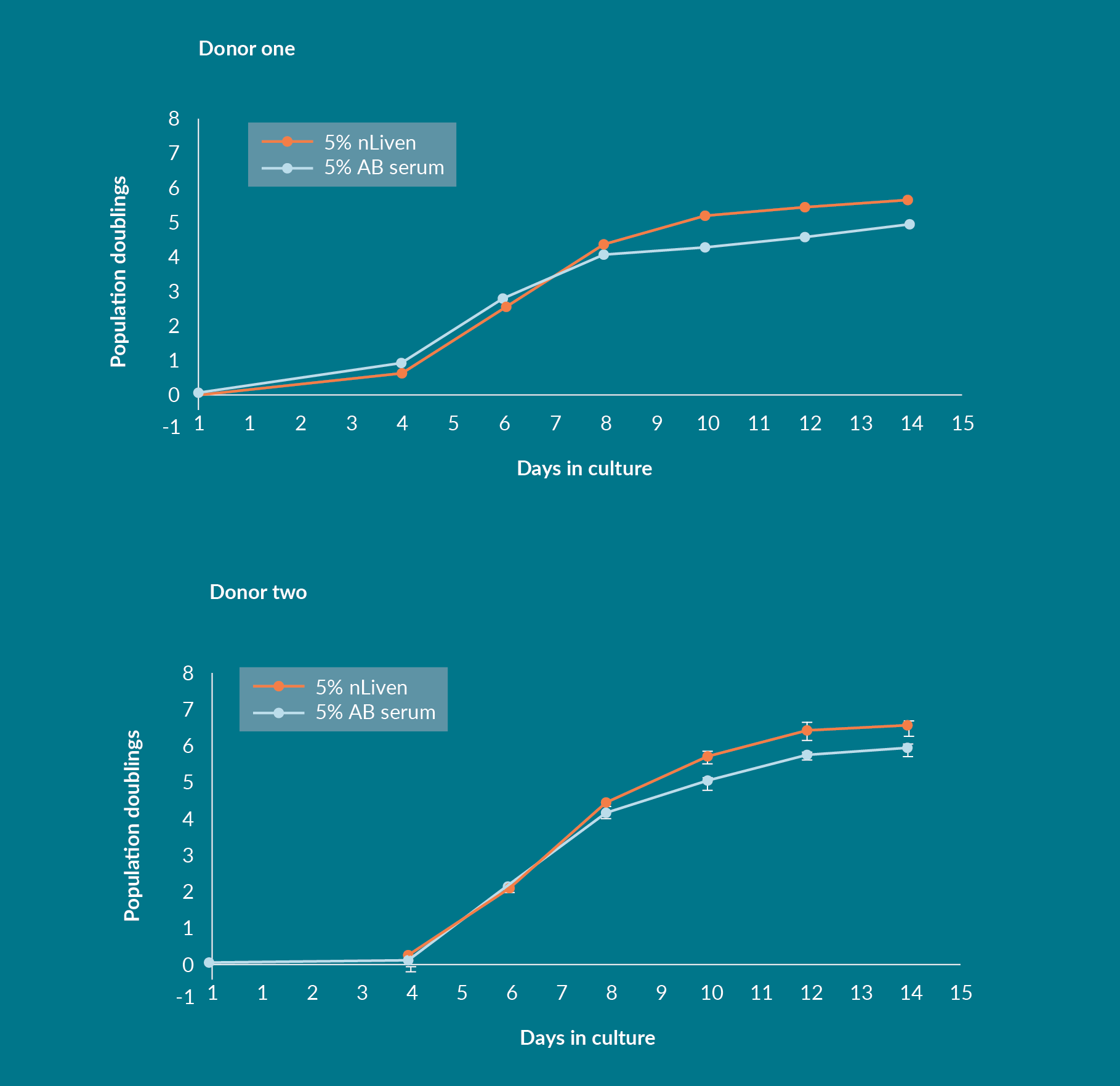
Negatively selected, homogenous CD3+ starting populations were obtained from three donors, activated with Dynabeads® (Thermofisher), and expanded for 11 days in stirred-tank bioreactors for the work performed at HCATS. This served to assess if the impact of using nLiven in place of standard protein supplements would persist in an agitated culture system as opposed to a static one. The average population doubling across all three donors was 6.1 ± 0.58 and 6.0 ± 1.37 for the nLiven PRTM and ABS conditions respectively (Figure 2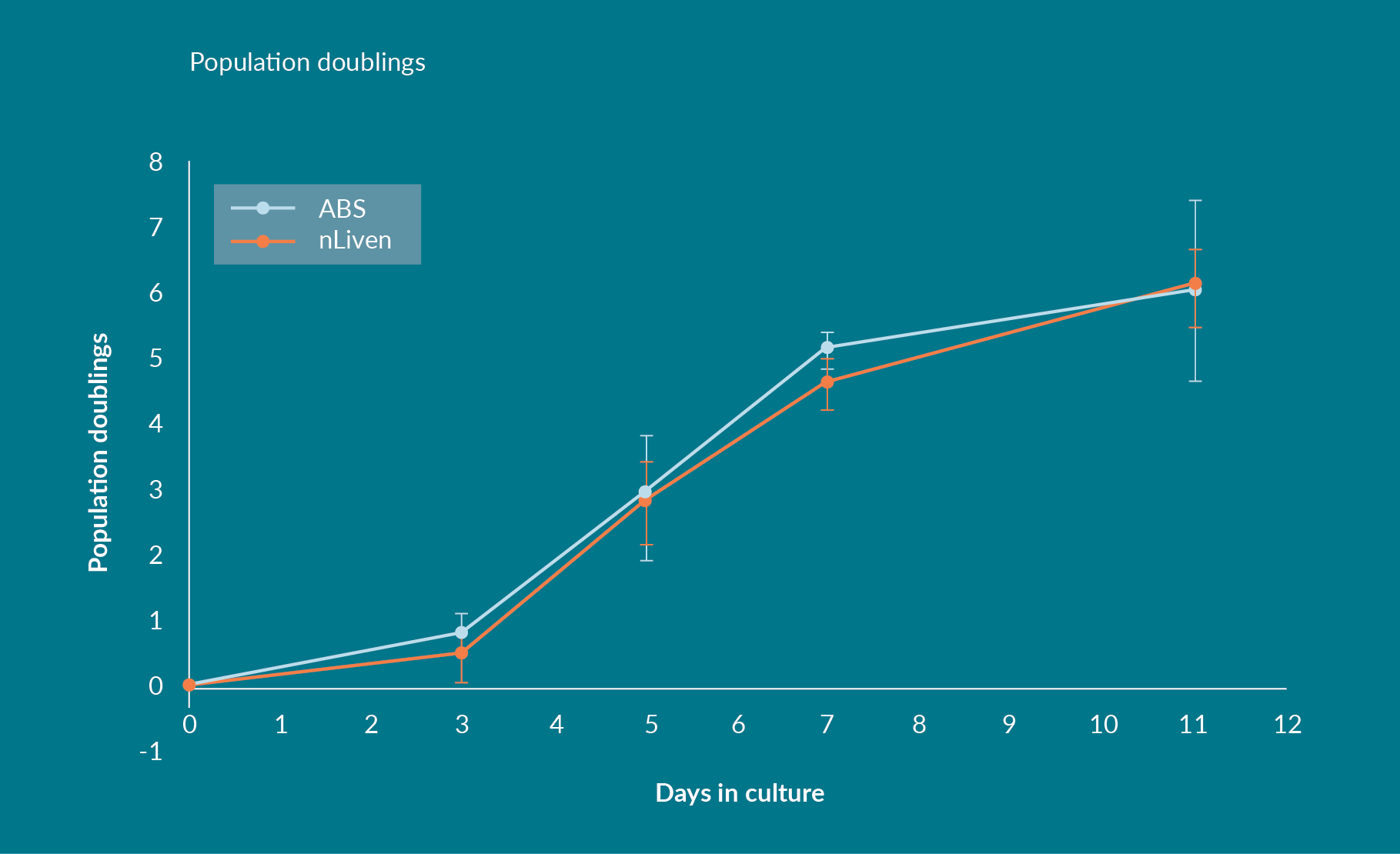
Process consistency is a persistent issue in ex vivo manufacture of human primary cells. Especially in autologous therapies, donor-to-donor variability forces a broadening of final product specifications and a looser understanding of critical quality attributes (CQAs). Changing to reagents that reduce variability is a powerful way to improve the understanding of those therapy characteristics. Developers may be able to produce comparable but better characterized therapies since variability was reduced in both studies when the media was supplemented with nLiven PRTM as opposed to human ABS and expansion remained consistent.
Cultures executed at both Sexton and HCATS would typically double between six and seven times during the culture period. Those results show that there was no negative impact on cell expansion by exchanging human ABS for nLiven PRTM and the substitution may result in an increase in total cell yield depending on the homogeneity of the starting cell population.
The percentage of a T-cell population that presents a central memory phenotype is typically negatively correlated to the number of times a population doubled. In two cultures that expanded a similar amount, for instance, the resulting TCM population should also be similar. What was exhibited when culturing with nLiven PRTM in static flasks, however, was a statistically significant increase in this TCM subset compared to media supplemented with ABS (Figure 3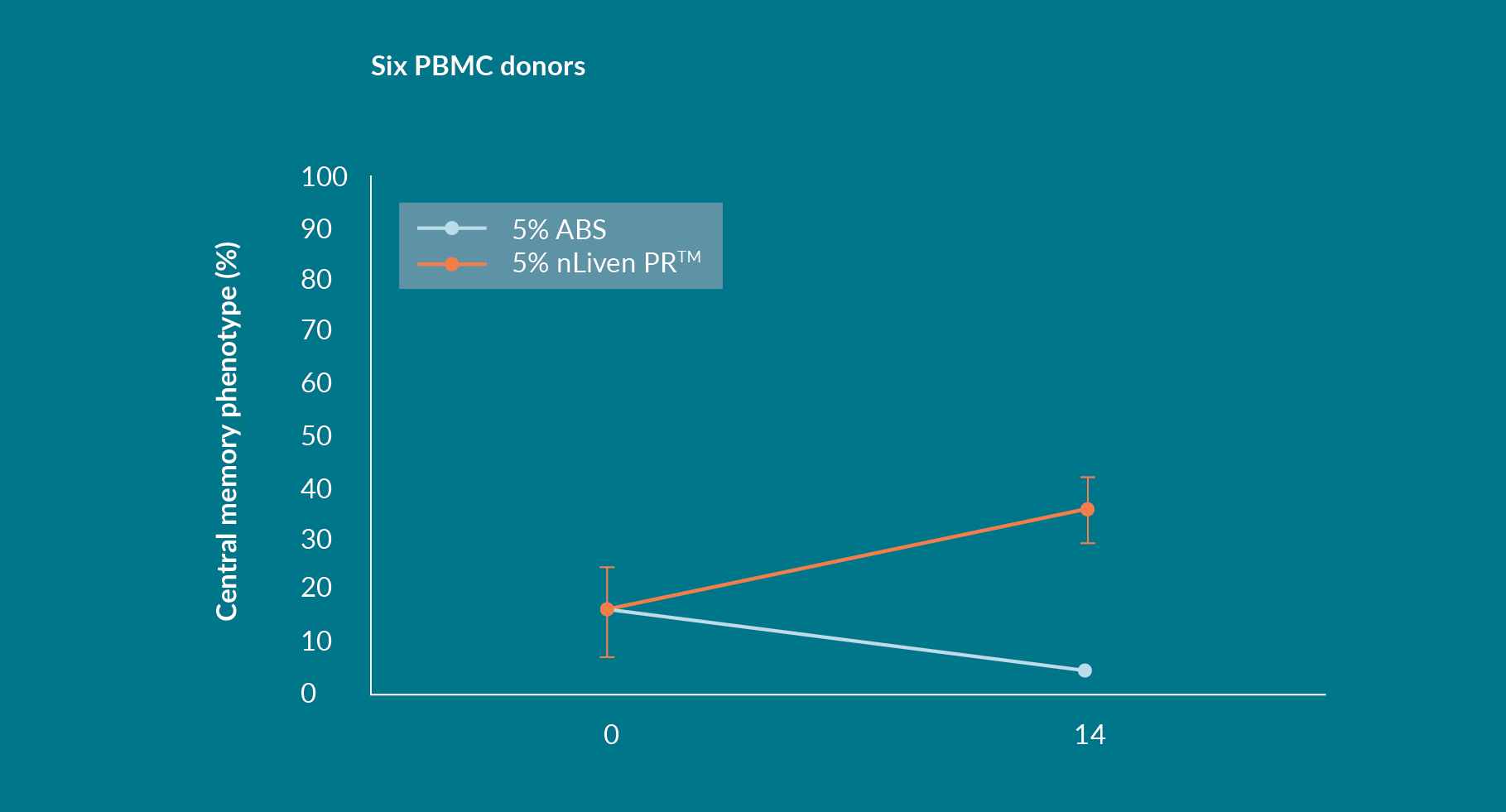
This analysis gives credence to the idea that yield should be evaluated as the resultant population of T cells with a favorable TCM phenotype. This quality cell yield could serve as a surrogate for lengthy and expensive in vivo potency assays that are out of reach for many groups without a vivarium or for imprecise and target-dependent ex vivo potency assays that do not account for the persistence of response. Evaluating culture performance by the final population of TCM may provide a more realistic determination of therapeutic relevance than total T-cell yield.
Regardless of the starting percentage of Central Memory T-Cells by the end of the 14-day culture period approximately 30% of the total population were classified as TCM – this seems to be a consistent and reproducible effect of growing PBMCs in nLiven PRTM. Equally, expanding PBMCs in ABS consistently results in a significant reduction of the TCM population.
Keeping with the results from Sexton’s experiments, the three donors tested by HCATS finished the culture period with about 50% of the T-cell population classified as TCM (Figure 4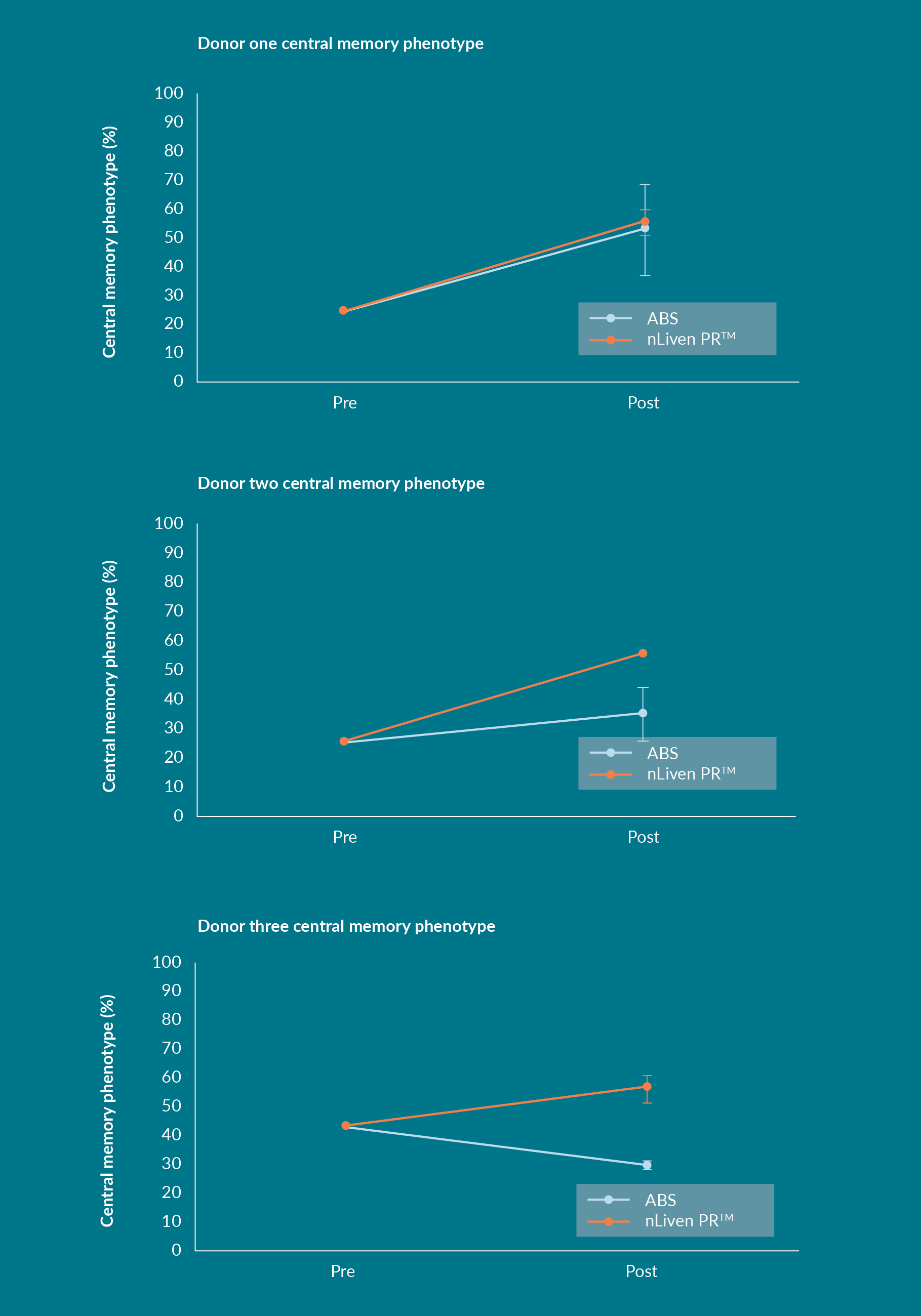
In both Sexton and HCATS studies the T cells cultured with nLiven PRTM saw a net increase in their TCM population after ex vivo expansion, which is contrary to the expectation that expanding primary, human T cells drives differentiation toward an effector phenotype. The same result was not duplicated by the cultures supplemented with human ABS. That suggests that the addition of nLiven PRTM selectively promotes the maintenance of a TCM phenotype throughout ex vivo cultures.
These independently conducted studies discovered a tendency for the memory phenotypes of the final T-cell population to favor subsets that were correlated with improved therapeutic efficacy and that the resulting expansion of the TCM populations were consistent between donors. In autologous therapies, final product specifications attempt to account for variation in starting material collected from individual patients, this factor was mirrored in these studies as Sexton’s starting populations were unpurified and HCATS studies utilized CD3+ selected cells. Regardless of starting material the final cell products showed remarkable similarities. Driving down lot-to-lot differences is a major consideration when developing an autologous manufacturing process, and nLiven PRTM is showing promise as a contributing factor to improved process robustness. Consistency itself is a valuable trait to develop into the production of an advanced therapy. As a derivative of human platelets, nLiven benefits from a more homogenous combination of biological chemicals than pooled human sera. This difference may be the reason for a more consistent response from primary, human cells.
Typically, the main consideration when evaluating a potential reagent change is the relative yield of viable cells throughout the process, and the results show a comparably high total expansion between the two protein supplements over the evaluated culture periods. However, there is an emerging mindset that focuses on attributes of cell quality in addition to bulk yield. For T cells, memory phenotypes can indicate a therapy’s ability to provide a persistent in vivo response. The resulting memory phenotypes trend toward the conclusion that TCM phenotypes are promoted in cultures that include nLiven PRTM in the media formulation. Multiple donors from two separate labs had significantly higher TCM populations post expansion. Consistency across a group of donors is another important consideration when investigating if a process impact can be translated into the clinic [7]Charlebois S, Canestrari E, Harris S. Characterization of a pathogen reduced human platelet lysate. Cytotherapy 2018; 20(5 Suppl.): S61.. When subjected to commercially relevant expansion nLiven PRTM supplemented media resulted in a lower level of variance in total cell yield or central memory phenotypes vs ABS – this indicates more consistent culture conditions that could translate to more robust product manufacturing. The results from the work performed at our labs lead to the conclusion that incorporating nLiven PRTM into T-cell manufacturing processes could lead to a high yield of quality therapeutic cells.
References
1. Klebanoff CA, Gattinoni L, Torabi-Parizi P et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. PNAS 2005; 102 (27): 9571–6. Crossref
2. Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. J. Exp. Med. 2005; 202(1): 123–33. Crossref
3. Hislop AD, Gudgeon NH, Callan MFC et al. EBV-Specific CD8+ T Cell Memory: Relationships Between Epitope Specificity, Cell Phenotype, and Immediate Effector Function. J. Immunol. 2001; 167(4): 2019–29. Crossref
4. Jackola DR, Ruger JK. Miller RA. Age-associated changes in human T cell phenotype and function. Aging Clin. Exp. Res. 1994; 6: 25–34.
5. “Blood Types.” NHS Blood Donation: www.blood.co.uk/why-give-blood/blood-types/ Crossref
6. Torres Chavez A, McKenna MK, Canestrari E et al. Expanding CAR T cells in human platelet lysate renders T cells with in vivo longevity. J. ImmunoTher. Cancer 2019; 7: 330. Crossref
7. Charlebois S, Canestrari E, Harris S. Characterization of a pathogen reduced human platelet lysate. Cytotherapy 2018; 20(5 Suppl.): S61. Crossref
Affiliations
Steven Thompson
Vice-President, Sales and Product Management, Sexton Biotechnologies
Alex Klarer
Hitachi Chemical Advanced
Therapeutics Solutions
David Smith
Hitachi Chemical Advanced
Therapeutics Solutions
Steve Charlebois
Vice President of Engineering,
Regenerative Medicine
MED Institute Inc.
Hayley Steidinger
Research Scientist, Regenerative Medicine, MED Institute Inc.
Amanda Taylor
Cook Research Incorporated,
1 Geddes Way, West Lafayette, Indiana
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: Dr. Klarer reports non-financial support from Cook Regentec, during the conduct of the study. Dr Thompson is a co-founder of Sexton Biotechnologies. Ms. Steidinger & Dr. Taylor have nothing to disclose.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2020 Sexton Biotechnologies. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited.
Submitted for peer review: Feb 19 2020; Revised manuscript received: Mar 16 2020; Publication date: Apr 8 2020.