Two new capture options for improved purification of large mRNA
Cell & Gene Therapy Insights 2020; 6(7), 1035–1046
10.18609/cgti.2020.114
One of the barriers to development of industrial purification platforms for large mRNA has been an inadequate selection of high-performing capture-purification tools. Hybridization-affinity uses a polythymidine (Oligo dT) ligand to base-pair with the polyadenine tail of mRNA. It can be used for capture but it cannot discriminate dsRNA (double-stranded) from ssRNA (single-stranded) and it supports only brief cleaning with 100 mM sodium hydroxide. Traditional anion exchangers elute only mRNA smaller than about 500 bases unless the columns are heated to 50–70°C. Hydrophobic interaction chromatography (HIC) and reverse phase chromatography (RPC) separate ssRNA from dsRNA and short transcripts, but their sensitivity to fouling by proteins and aggregates makes them better suited for polishing than for capture. Better capture options are needed to meet the needs of large clinical trials, scale-up, and manufacture of vaccines. Beyond that, a new spectrum of gene therapy treatments await. This article introduces two new capture options that both eliminate dsRNA, DNA, and proteins in a wash step, then provide high-resolution polishing of ssRNA in an elution gradient at ambient temperature. One represents a new class of anion exchangers. The other exploits hydrogen bonding. Both support prolonged exposure to 1 M sodium hydroxide. Easy transition to either HIC or RPC provides high-resolution orthogonal polishing.
Introduction
One of the barriers to development of industrial purification for large mRNA has been an inadequate selection of high-performing capture-purification tools. Hybridization-affinity uses a polythymidine (Oligo dT) ligand to base-pair with the polyadenine tail of mRNA. It can be used for capture but it cannot discriminate dsRNA (double-stranded) from ssRNA (single-stranded) and it supports only brief cleaning with 100 mM sodium hydroxide [1]Satterfield B, Stern S, Caplan M, Hukari K, West J. Microfluidic purification and preconcentration of mRNA by flow-through polymeric monolith. Anal. Chem. 2007; 79: 6230–35., [2]Slater R. The purification of poly(A)-containing RNA by affinity chromatography. Met. Molec. Biol. 1984; 2: 117–20., [3]Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. . Ambient temperature operation of traditional anion exchangers elutes only mRNA species smaller than about 500 bases [3]Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. , [4]Koubek J, Lin KF, Chen YR, Cheng RP, Huang JJT. Strong anion-exchange fast performance liquid chromatography as a versatile tool for preparation and purification of RNA produced by in vitro transcription. RNA 2013; 19: 1449–59., [5]Prazeres D, Schluep T, Coony C. Preparative purification of supercoiled plasmid DNA using anion exchange chromatography, J. Chromatogr. A 1998; 806: 31–4. Elution of larger species requires elevation of operating temperature into the range of 50–70°C [6]Issa W, Barberio J, Aunins J, Afeyan N. Ion exchange purification of mRNA, World patent application WO2014144767A1, priority date March 15, 2013.. Hydrophobic interaction chromatography (HIC) and reverse phase chromatography (RPC) separate ssRNA from DNA, dsRNA, and short transcripts, but their sensitivity to fouling by proteins and aggregates makes them better suited for polishing than for capture [3]Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. , [7]Kariko K, Muramatsu H, Ludwig J, Weismann D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acid Res. 2011; 39: el42.Kariko K, Muramatsu H, Ludwig J, Weismann D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acid Res. 2011; 39: el42., [8]Weismann D, Pardi N, Murumatsu H, Kariko K. HPLC purification of in vitro transcribed long RNA. Met. Molec. Biol. 2013; 969 43–54.Weismann D, Pardi N, Murumatsu H, Kariko K. HPLC purification of in vitro transcribed long RNA. Met. Molec. Biol. 2013; 969 43–54., [9]Azarani A, Hecker K. RNA analysis by ion-pair reversed-phase high performance liquid chromatography. Nucl. Acid. Res. 2001; 29: e7.Azarani A, Hecker K. RNA analysis by ion-pair reversed-phase high performance liquid chromatography. Nucl. Acid. Res. 2001; 29: e7., [10]Majors R. The cleaning and regeneration of reversed-phase HPLC columns, LC-GC Europe, July 2003: https://www.chemass.si/ca_content/Cleaning_and_maintaining_C-18_columns.pdfMajors R. The cleaning and regeneration of reversed-phase HPLC columns, LC-GC Europe, July 2003: https://www.chemass.si/ca_content/Cleaning_and_maintaining_C-18_columns.pdf.
Better capture options are needed to meet the needs of large clinical trials, scale-up, and manufacture of vaccines. Beyond that, a new spectrum of gene therapy treatments await. This article introduces two new capture options that both eliminate dsRNA, DNA, and proteins in a wash step, then provide high-resolution polishing of ssRNA in an elution gradient at ambient temperature. One represents a new class of anion exchangers. The other exploits hydrogen bonding. Both support prolonged exposure to 1 M sodium hydroxide. Easy transition to either HIC or RPC provides high-resolution orthogonal polishing.
Experimental
CIMac™ (100 µL) or CIMmultus™ (1 mL) PrimaS™ and H-Bond™ monoliths with 2 µm channels were obtained from BIA Separations. Single-stranded and dsRNA ladders, DNA ladders, and species of single size were obtained from New England Biolabs. Analytical grade or American Chemical Society grade buffering agents and salts were obtained from Sigma-Aldrich. Buffers were prepared fresh with European Pharmacopeia grade water and filtered to 0.22 µm before use.
Purified samples of defined content were used to eliminate ambiguity of interpretation and facilitate comparison across laboratories. They were equilibrated before injection by dilution with a 10-fold volumetric excess of the column equilibration buffer. Many examples were performed with sample mixtures containing supercoiled DNA of 6000 base pairs and ssRNA of 5000 bases. Injection volumes ranged from 50 µL to 200 µL depending on the size of the column. Specific sample composition and buffer conditions are described in the Figure legends. Columns were operated at a flow rate of 5 column volumes per minute (300 CV/h). Results obtained from experiments with conditions and samples of broader scope are described in [3]Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. .
RNA capture by anion exchange chromatography, elution by pH gradient
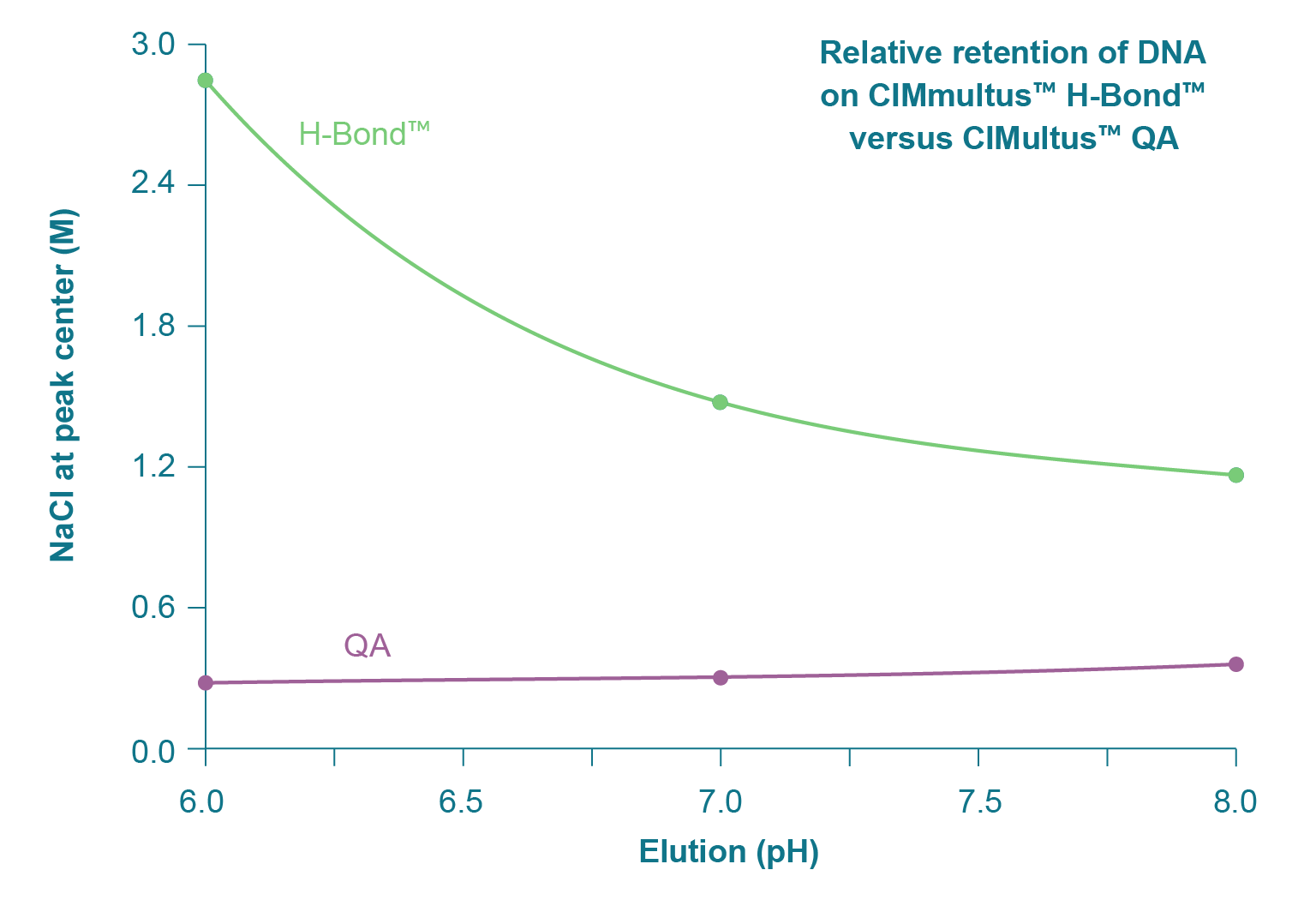
The necessity to heat traditional anion exchangers represents a burden at all stages of process development and manufacturing but it also provides a clue. The inability to elute large mRNA at ambient temperature derives from the elevated hydrogen bonding capacity of RNA [3]Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. . The ratio of hydrogen donors and acceptors to negatively charged phosphatidic residues on the polymer backbone is more than 20:1 (Figure 1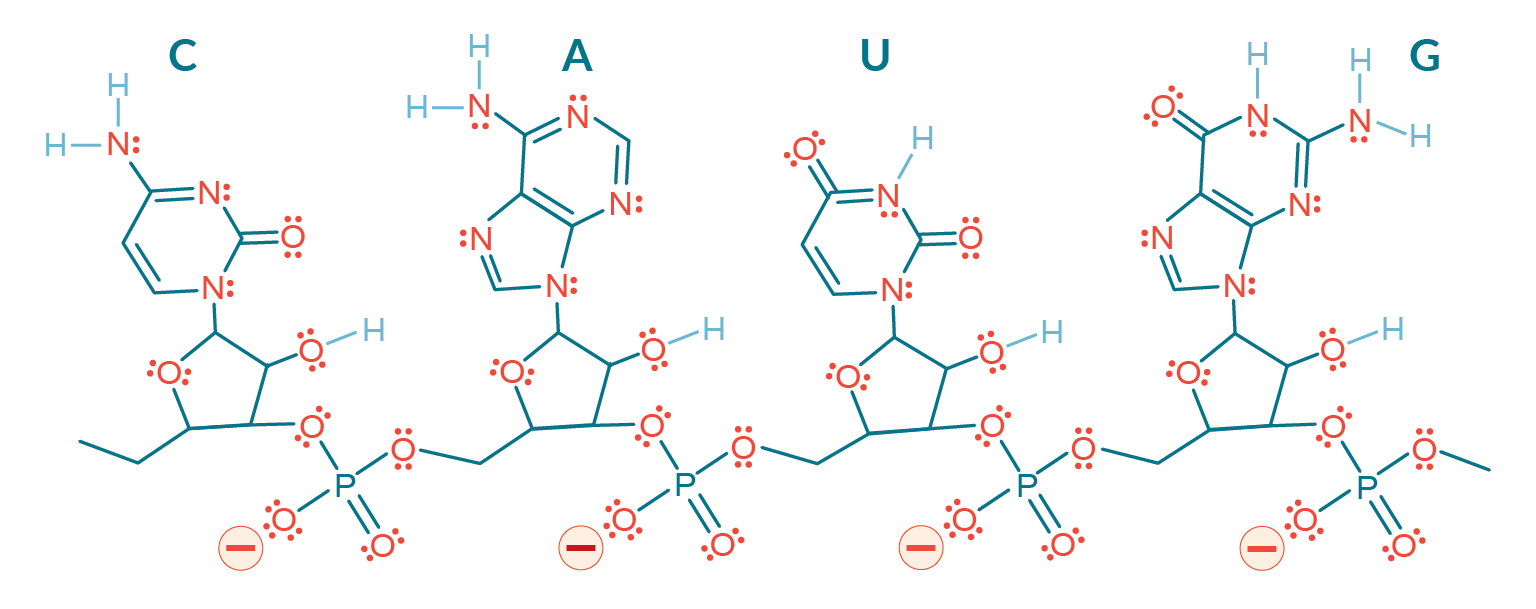
Figure 2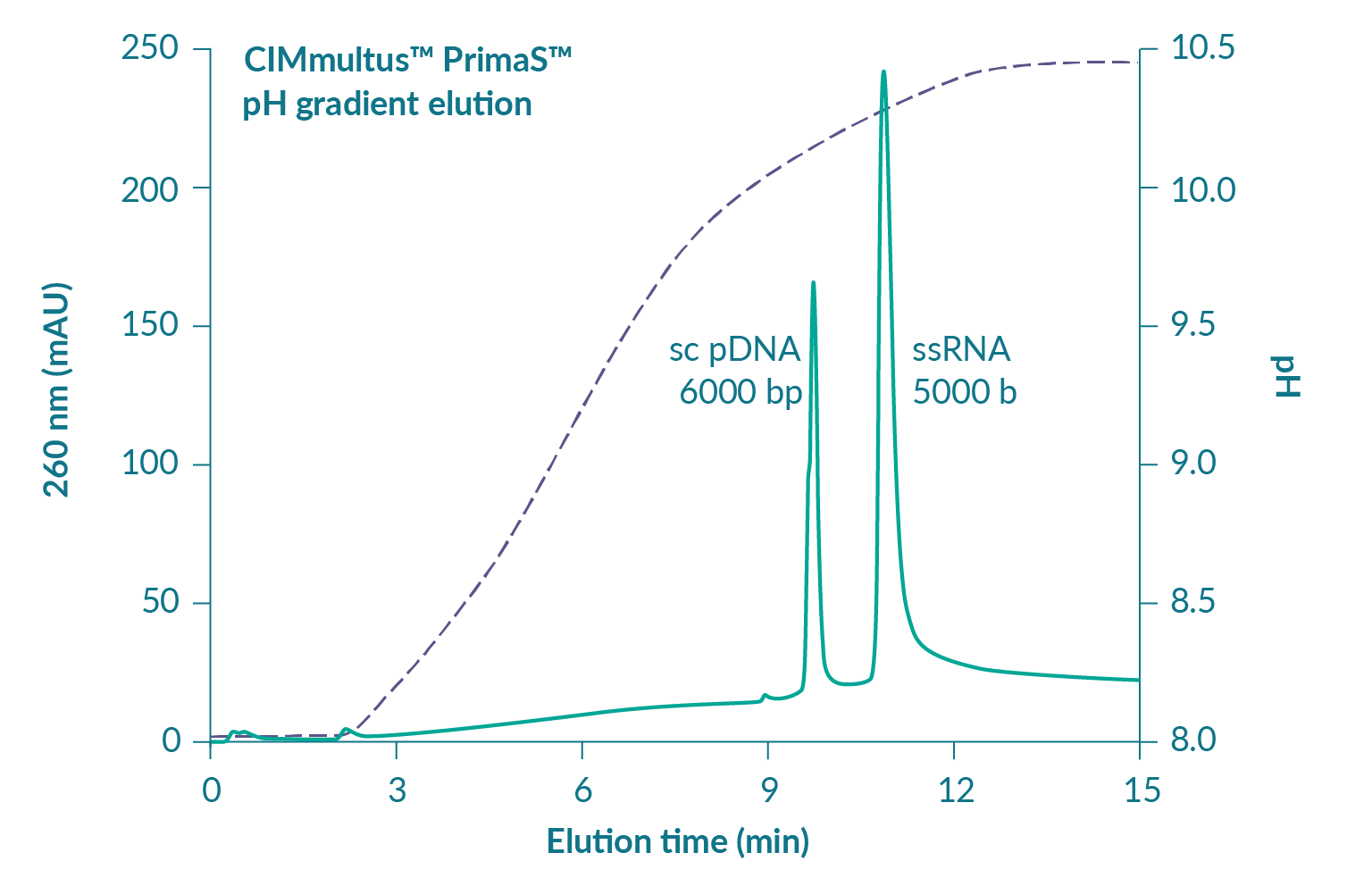
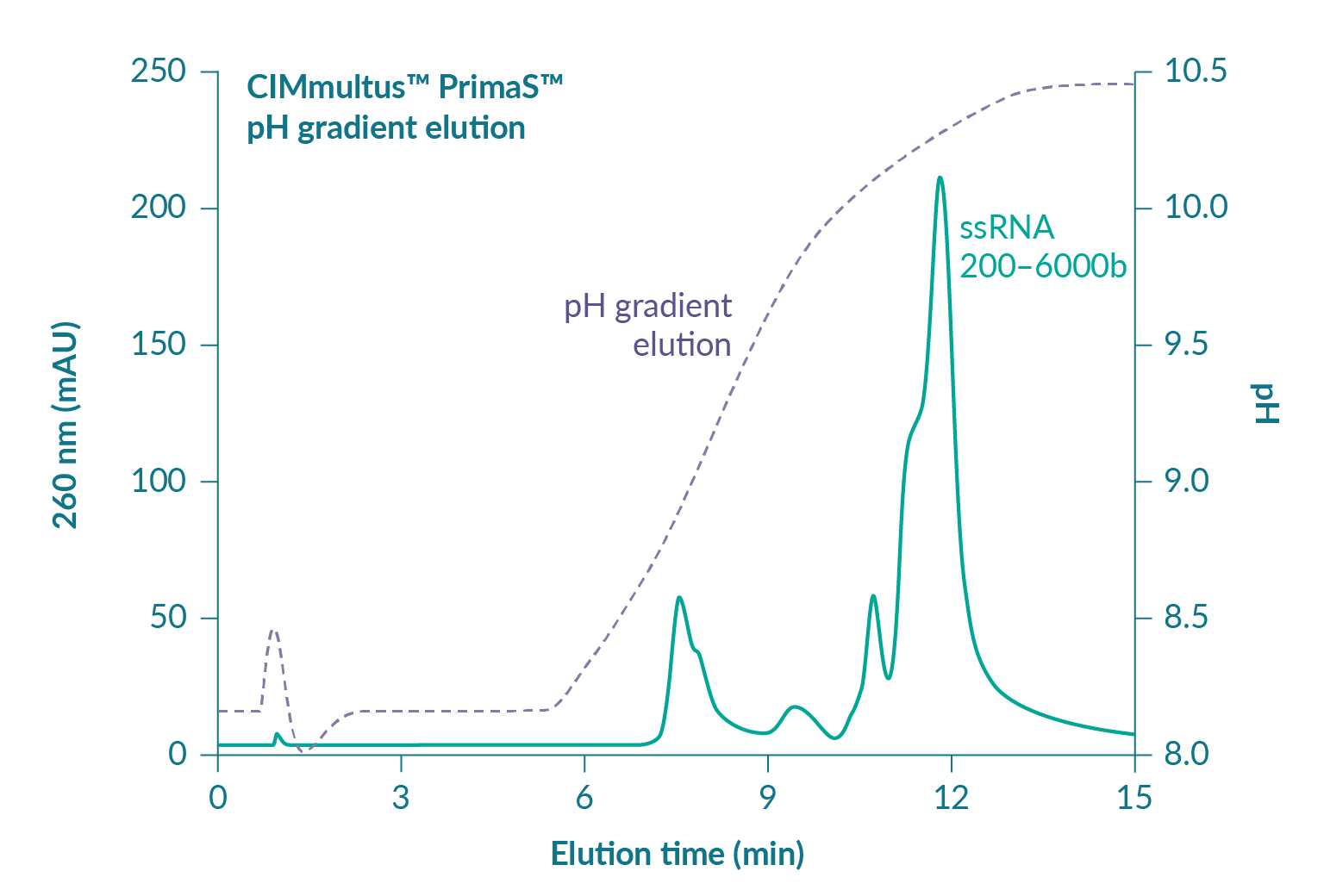
Contaminating double-stranded nucleic acids, including both DNA and dsRNA, are serious concerns from an immunological perspective [13]Liu M. A comparison of plasmid DNA and mRNA as vaccine technologies. Vaccines 2019; 7: 37.. Residual plasmid DNA is immunogenic [14]Yoon S, Seo Y, Im S et al. Safety and immunogenicity of therapeutic DNA vaccine with antiviral drug in chronic HBV patients and its immunogenicity in mice. Liver Int. 2015; 35: 805–15., [15]Grifantini R, Finco O, Bartolini E et al. Multi-plasmid DNA vaccination avoids antigenic competition and enhances immunogenicity of a poorly immunogenic plasmid. Eur. J. Immunol. 1998; 28: 1225–32. and may be present in range of degradation states at 1–2% of total RNA after transcription. Proportions of dsRNA are less well characterized but still important. Cells interpret dsRNA as a viral infection [16]Gantier M, Williams B. The response of mammalian cells to double-stranded RNA. Cytokine Growth Factor Rev. 2007; 18: 363–71.. It can trigger a cytokine storm with sudden and serious health consequences. Figure 4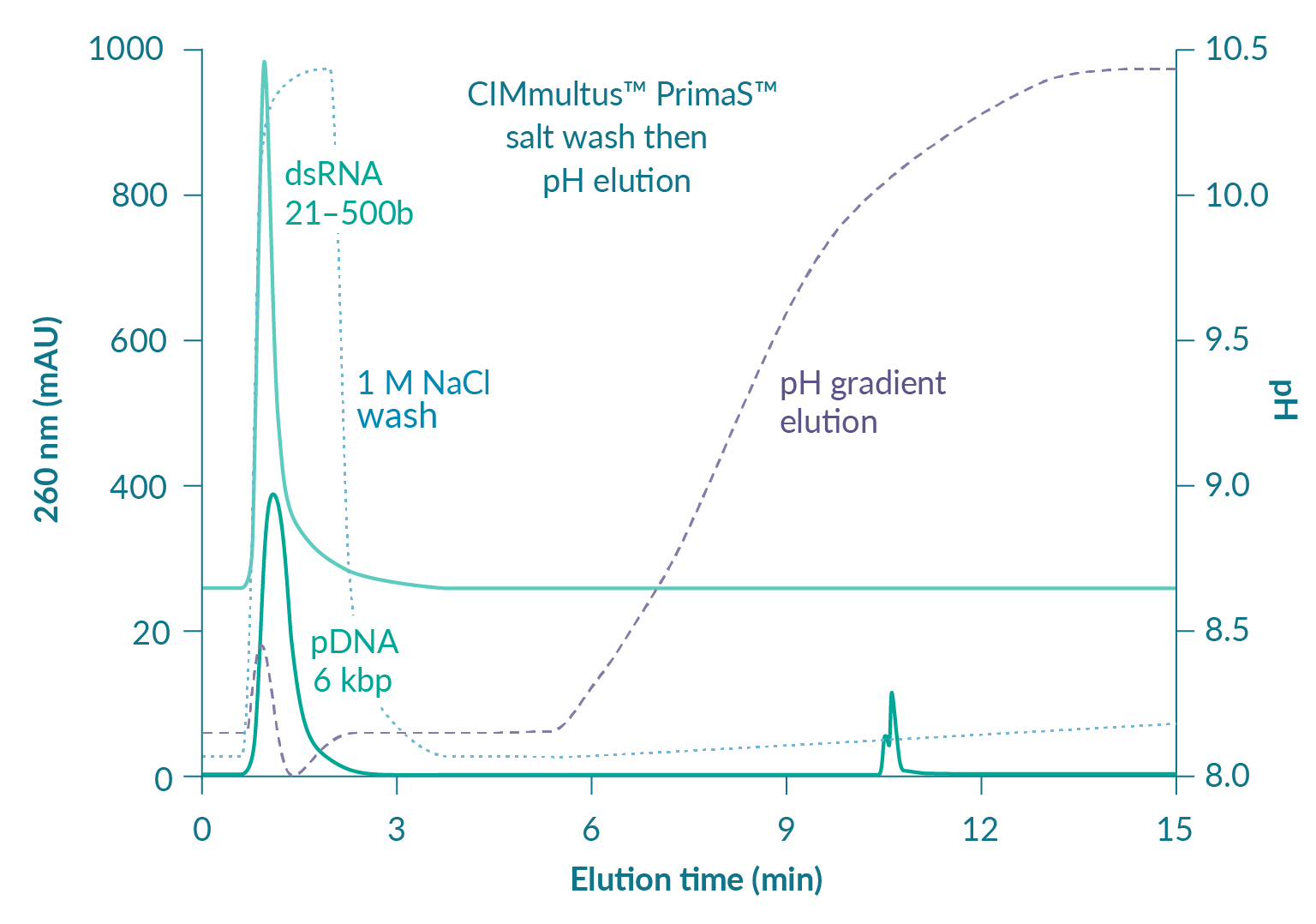
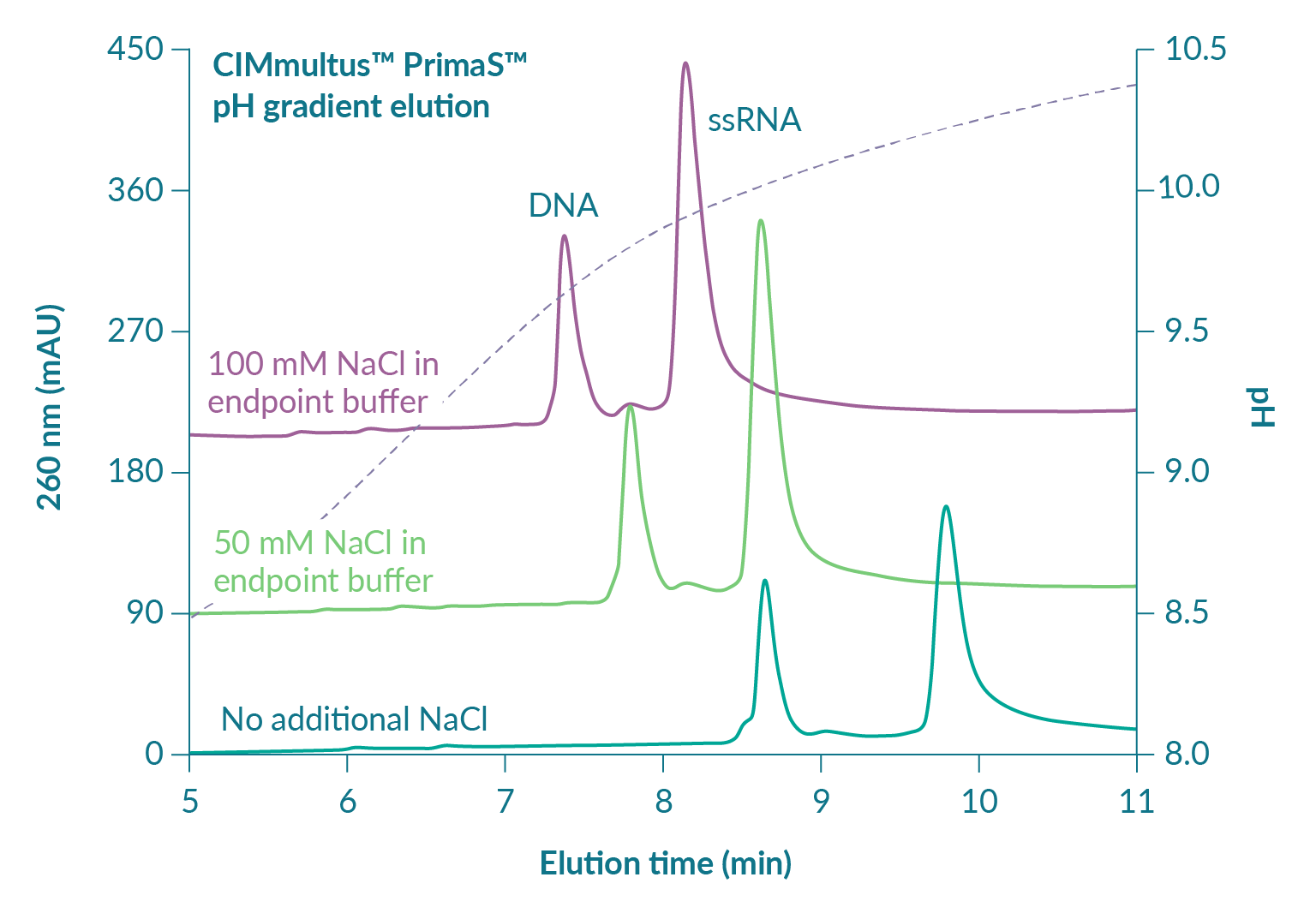
The pH of the eluted ssRNA should be neutralized during or shortly after elution. Both methods are standard practice in the field of protein affinity chromatography where fraction collection vessels either contain a pH-titrating buffer so the product is neutralized upon collection, or the product is neutralized at the end of the run. Preliminary data indicate that brief exposure to alkaline elution conditions causes no modification of mRNA but prudence suggests avoiding prolonged exposure.
The column can be cleaned extensively with 1 M NaOH. Brief cleaning is recommended after every run since it will reveal the amount of material remaining on the column after elution. Columns loaded with large volumes of crude samples may require cleaning for 1 hour. Badly fouled columns can be restored to baseline performance by cleaning for 16–24 hours. Cleaning can be enhanced by co-formulating NaOH with 1–3 M NaCl and 10–20 mM EDTA.
RNA capture by hydrogen bonding, affinity elution by diphosphate displacement
Figure 6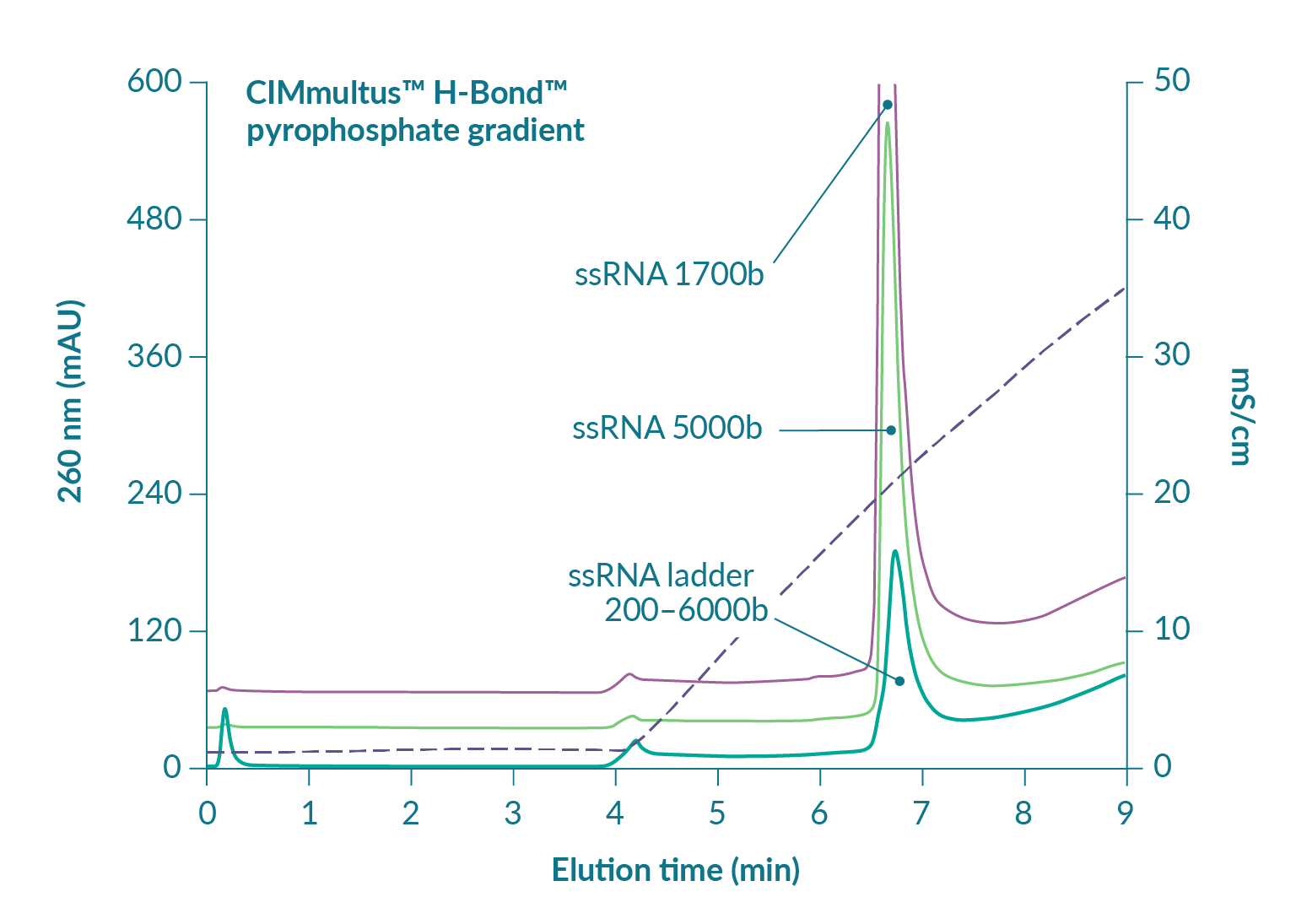
Pyrophosphate is a diphosphate (P2O7) with up to 4 negative charges and up to 18 hydrogen donor/acceptors depending on pH (Figure 8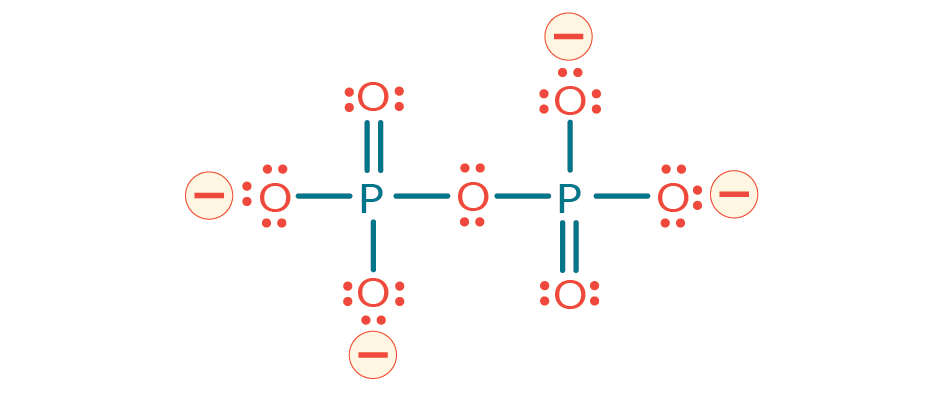
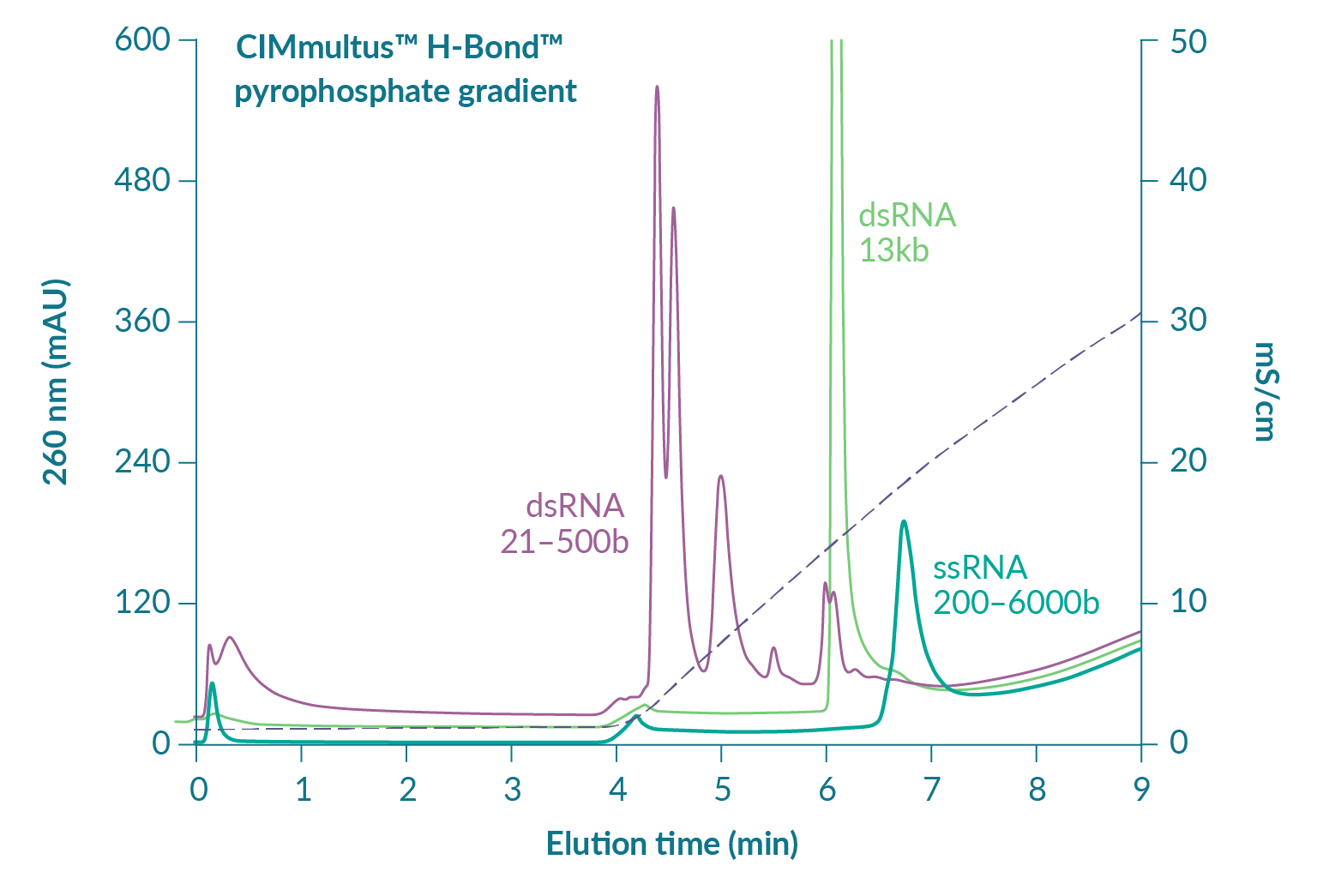
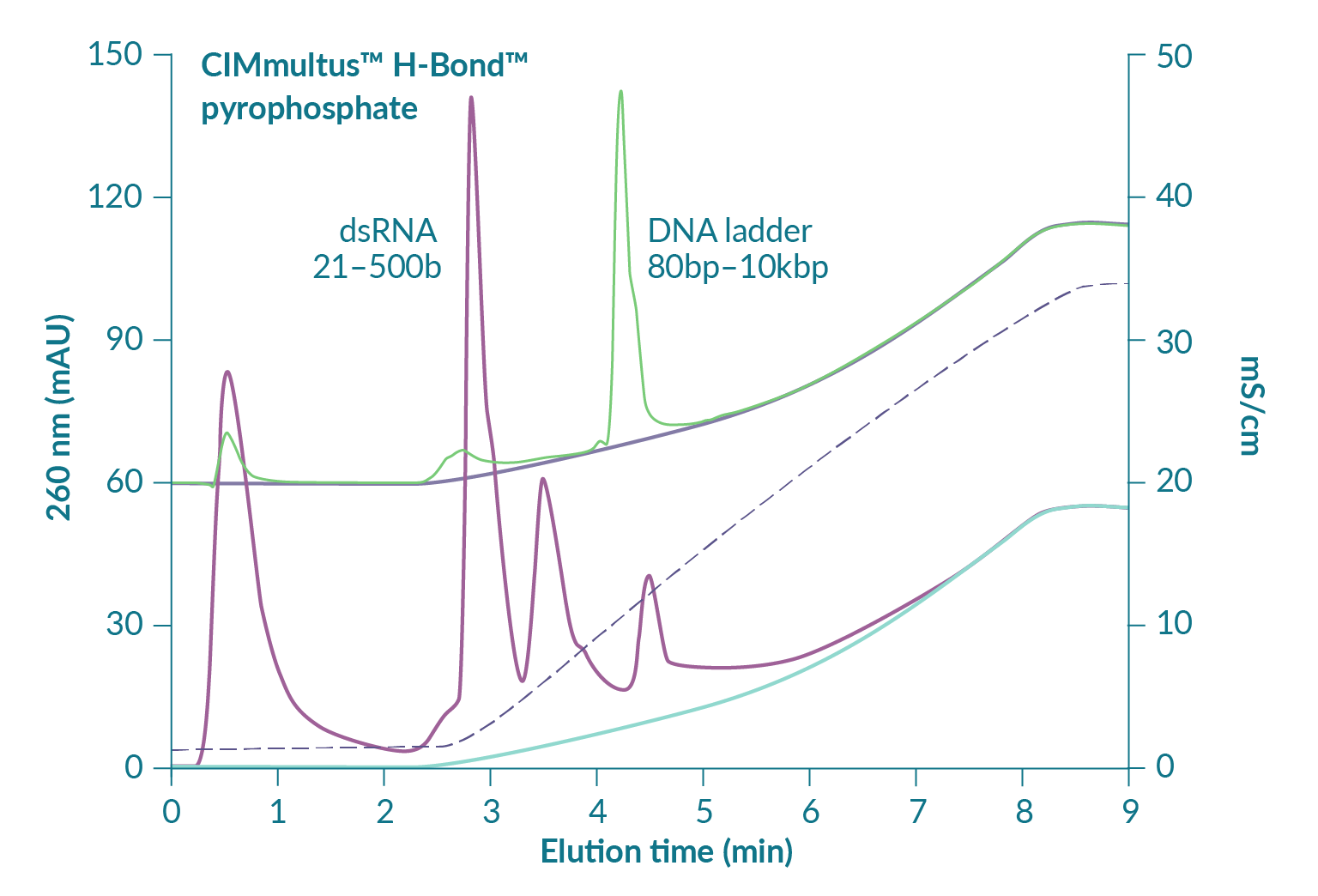
Figure 7 suggests that reducing pH will increase RNA capacity but also predicts that elution of ssRNA will be shifted to a higher concentration of pyrophosphate [3]Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. . It does not necessarily follow that separation between ssRNA and double-stranded contaminants will remain the same but that concern can be managed in a different way. As with anion exchange chromatography, DNA and dsRNA are eliminated at neutral pH by a wash step with 1 M NaCl and 10 mM EDTA [3]Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. . At lower pH, increasing the salt concentration in the wash should compensate for stronger binding and leave the gradient to polish out trace-level contaminants from highly purified ssRNA.
Running the gradient at alkaline pH and/or in the presence of non-pyrophosphate salts elutes ssRNA at lower pyrophosphate concentrations. Combinations of other salts and alkaline pH can elute ssRNA without pyrophosphate but only pyrophosphate elution separates dsRNA from ssRNA. Optimization parameters and ranges are discussed in detail in [3]Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. . H-Bond supports the same robust NaOH tolerance as PrimaS™.
Pyrophosphate anions must be removed from the final product because, in vivo, they form precipitates with calcium that can cause adverse health consequences. Pyrophosphate has the same charge as RNA, which suggests they should repel each other. However, their shared metal affinity creates potential for them to form stable coordination bonds via multivalent metal cations [3]Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. . A chelating agent needs to be present at a significant concentration. Pyrophosphate removal can be performed during final formulation by diafiltration but doing it in the context of a polishing step achieves the goal earlier in the process and increases confidence that it will be absent from the final product. Sensitive pyrophosphate assays to validate clearance are available from global suppliers.
Polishing after capture
Coming out of a capture step with highly purified ssRNA, particularly lacking in double-stranded nucleic acids, contributes robustness to purification platforms using two chromatography steps. Removing the majority of DNA and dsRNA in advance allows the polishing step to accomplish what it is intended to do: polish. This is substantially preferable to the alternative of coming from capture with virtually the entire load of the most toxic contaminants at full strength, then relying on a single polishing step to fully remove them.
Otherwise, polishing may employ the same options used after capture by hybridization-affinity chromatography. HIC or RPC each provide independent orthogonal ability to separate ssRNA from dsRNA, DNA, and proteins, and each achieves a degree of size separation to remove short transcripts. RPC gives better resolution than HIC but it requires the use of flammable solvents at elevated operating temperatures. RPC can be performed with either styrenedivinylbenzene (SDVB) or C-18 media but only SDVB is cleanable with 1 M NaOH [3]Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. , [7]Kariko K, Muramatsu H, Ludwig J, Weismann D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acid Res. 2011; 39: el42.Kariko K, Muramatsu H, Ludwig J, Weismann D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acid Res. 2011; 39: el42., [8]Weismann D, Pardi N, Murumatsu H, Kariko K. HPLC purification of in vitro transcribed long RNA. Met. Molec. Biol. 2013; 969 43–54.Weismann D, Pardi N, Murumatsu H, Kariko K. HPLC purification of in vitro transcribed long RNA. Met. Molec. Biol. 2013; 969 43–54., [9]Azarani A, Hecker K. RNA analysis by ion-pair reversed-phase high performance liquid chromatography. Nucl. Acid. Res. 2001; 29: e7.Azarani A, Hecker K. RNA analysis by ion-pair reversed-phase high performance liquid chromatography. Nucl. Acid. Res. 2001; 29: e7., [10]Majors R. The cleaning and regeneration of reversed-phase HPLC columns, LC-GC Europe, July 2003: https://www.chemass.si/ca_content/Cleaning_and_maintaining_C-18_columns.pdfMajors R. The cleaning and regeneration of reversed-phase HPLC columns, LC-GC Europe, July 2003: https://www.chemass.si/ca_content/Cleaning_and_maintaining_C-18_columns.pdf.
HIC-polishing after either anion exchange or hydrogen bond chromatography enables exclusively ambient aqueous purification. In place of hazardous materials and conditions, HIC imposes a lesser logistical burden. Binding ssRNA to HIC media requires high concentrations of salts to drive retention. Those salts promote precipitation of RNA. If the RNA precipitates before it reaches the binding surfaces inside the column, those precipitates interfere with sample loading, they depress capacity, and they depress purification performance.
This challenge was resolved decades ago for preparative HIC purification of proteins. It requires sample loading by a technique known as in-line dilution. In-line dilution requires two input lines that meet at a mixer immediately before the column. This reduces the pre-column residence time of the ssRNA in high-salt to seconds, which prevents formation of large precipitates that would negatively affect chromatography. In addition, pre-column residence time of the sample in high salt remains uniform throughout the entire sample application phase, no matter how large the volume and how long it takes to load. Capacity and purification performance both benefit.
The first input line carries either low-salt buffer or sample, with an in-line 3-way valve to select one or the other. The second input line carries a high-salt diluent. Column equilibration is conducted with a mix of low-salt buffer with the high-salt diluent, for example 4 parts diluent to 1 part low-salt buffer. Sample application is done by switching the valve to deliver sample at the same mixing proportion. A wash step is performed by switching the valve back to low-salt buffer. Elution is performed by reproportioning the high and low salt buffers. HIC sample-loading by-inline dilution is discussed in detail in [3]Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. .
All of these options support smooth workflow. The low salt concentration of ssRNA after pH elution from PrimaS™ simplifies sample preparation going into the low-no salt method of RPC. After either anion exchange or hydrogen bonding chromatography, applying the sample to HIC simply requires adding salt. Inclusion of EDTA in the HIC binding salt helps displace residual pyrophosphates and it is also good insurance to eliminate residual metal ions carried over from any previous step.
Conclusions
Anion exchange and hydrogen bond chromatography can both be used to prepare research quality ssRNA in a single step. More importantly, they both provide an improved capture-foundation for two-step purification of clinical-quality single-stranded mRNA. Thanks to their ability to largely eliminate dsRNA and DNA with a salt wash, linear gradient elution can be converted to a step format with little or no compromise to purification of ssRNA. Both methods reduce the overall contaminant load going into polishing and they enhance robustness of the platform overall. Both methods support aggressive cleaning and sanitization with sodium hydroxide to enable multiple use, and both support a full range of scale-up options.
References
1. Satterfield B, Stern S, Caplan M, Hukari K, West J. Microfluidic purification and preconcentration of mRNA by flow-through polymeric monolith. Anal. Chem. 2007; 79: 6230–35. Crossref
2. Slater R. The purification of poly(A)-containing RNA by affinity chromatography. Met. Molec. Biol. 1984; 2: 117–20. Crossref
3. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Crossref
4. Koubek J, Lin KF, Chen YR, Cheng RP, Huang JJT. Strong anion-exchange fast performance liquid chromatography as a versatile tool for preparation and purification of RNA produced by in vitro transcription. RNA 2013; 19: 1449–59. Crossref
5. Prazeres D, Schluep T, Coony C. Preparative purification of supercoiled plasmid DNA using anion exchange chromatography, J. Chromatogr. A 1998; 806: 31–45. Crossref
6. Issa W, Barberio J, Aunins J, Afeyan N. Ion exchange purification of mRNA, World patent application WO2014144767A1, priority date March 15, 2013. Crossref
7. Kariko K, Muramatsu H, Ludwig J, Weismann D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acid Res. 2011; 39: el42. Crossref
8. Weismann D, Pardi N, Murumatsu H, Kariko K. HPLC purification of in vitro transcribed long RNA. Met. Molec. Biol. 2013; 969 43–54. Crossref
9. Azarani A, Hecker K. RNA analysis by ion-pair reversed-phase high performance liquid chromatography. Nucl. Acid. Res. 2001; 29: e7. Crossref
10. Majors R. The cleaning and regeneration of reversed-phase HPLC columns, LC-GC Europe, July 2003: https://www.chemass.si/ca_content/Cleaning_and_maintaining_C-18_columns.pdf Crossref
11. Pabst T, Carta G, Ramasubramanyan N, Hunter A, Mensah P, Gustafson M. Separation of protein charge variants with induced pH gradients using anion exchange chromatography columns. Biotechnol. Prog. 2008; 24: 1096–106. Crossref
12. Sluyterman L, O Elgersma O. Chromatofocusing: isoelectric focusing on ion-exchange columns: I. General principles. J. Chromatogr. A 1978; 150: 17–30. Crossref
13. Liu M. A comparison of plasmid DNA and mRNA as vaccine technologies. Vaccines 2019; 7: 37. Crossref
14. Yoon S, Seo Y, Im S et al. Safety and immunogenicity of therapeutic DNA vaccine with antiviral drug in chronic HBV patients and its immunogenicity in mice. Liver Int. 2015; 35: 805–15. Crossref
15. Grifantini R, Finco O, Bartolini E et al. Multi-plasmid DNA vaccination avoids antigenic competition and enhances immunogenicity of a poorly immunogenic plasmid. Eur. J. Immunol. 1998; 28: 1225–32. Crossref
16. Gantier M, Williams B. The response of mammalian cells to double-stranded RNA. Cytokine Growth Factor Rev. 2007; 18: 363–71. Crossref
17. Cole R. The chromatography of insulin in a urea-containing buffer. J. Biol. Chem. 1960; 235: 2294–9. Crossref
18. Cole R. Ion exchange chromatography of prolactin in urea-containing buffers. J. Biol. Chem. 1961; 236: 1369–71. Crossref
19. Fujita T, Suzuki Y, Yamauti JI et al. Chromatography in the presence of high concentrations of salts on columns of celluloses with and without ion exchange groups (hydrogen bond chromatography): its application to purification of yeast enzymes. J. Biochem. 1980; 87: 89–100. Crossref
20. Parente E, Wetlaufer D. Influence of urea on high performance cation exchange chromatography of hen egg white lysozyme. J. Chromatogr. A 1984; 288: 389–98. Crossref
Affiliations
Pete Gagnon
CSO, BIA Separations
Blaž Goričar
Project Manager, BIA Separations
Špela Peršič
Research Scientist in Process
Analytics Development,
BIA Separations
Urh Černigoj
Senior Scientist, BIA Separations
Aleš Štrancar
CEO, BIA Separations
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: Some of the figures in this article were adapted from [3]Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. Gagnon P. Purification of Nucleic Acids: A handbook for purification of plasmid DNA and mRNA for gene therapy and vaccines. BIA Separations, Ajdovščina, 2020; 136. with permission from the publisher.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2020 BIA Separations. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Jul 24 2020; Revised manuscript received: Aug 20 2020; Publication date: date.

