Limiting variability to achieve reproducibility in cell manufacturing
Cell & Gene Therapy Insights 2020; 6(10) 1357–1363
10.18609/cgti.2020.149
Cellular raw material is a primary source of variability in autologous cell therapy manufacturing and the inherent differences in donor apheresis products can impact the success of generating a reproducible final product. Standardization of apheresis collection methods coupled with a responsive manufacturing process will help to ensure reproducibility of the final product with variable input but presents a challenge in process standardization. A more thorough understanding of appropriate measures to evaluate and demonstrate product and process control will help to guide future improvements in product quality and manufacturing efficiency.
Over the past four decades, traditional biopharmaceutical manufacturers have made incredible advances in manufacturing platforms and facility design. The integration of six sigma manufacturing principles and a quality by design (QbD) approach has enabled the development of highly defined and optimized processes. The advancements in place have come from the standardization of starting material (e.g. cell lines, cell-free systems), innovative engineering technology and an influx of vendors providing ancillary materials solutions. However, even 15 years ago, the industry struggled in much the same way we struggle with cell and gene therapy manufacturing now. Since 2003, BioPlan Associates, Inc. has conducted an annual global survey of biopharmaceutical professionals. The survey report captures responses from more than 200 representatives in the biopharmaceutical manufacturing industry and over 130 suppliers regarding the current state of manufacturing challenges, production capacity, current trends in technology and resource planning. In 2018, a report was issued summarizing the key shifts in responses compared to the first report 15 years earlier. Not surprisingly, respondents indicated that developments in upstream processing, greater standardization and expanded access to trained staff have driven capacity improvements over the past decade [1]Rader R, Langer E. Fifteen Years of Progress: Biopharmaceutical Industry Survey Results. Pharmaceutical Technology. 2018; 42(7): 56–9..
We now find ourselves in an analogous position for autologous cell and gene therapy (CGT) manufacturing. If we hope to achieve reproducible results, meaning final products that consistently meet quality standards and specifications, then improvements are needed to effectively reduce product and process variability. Unfortunately, autologous cell manufacturing is plagued by variability at many stages in the process. A primary source of variability is introduced by donor to donor differences in autologous starting material and so a standardized process does not ensure a reproducible product. It follows that purity of the starting material, fresh or frozen, or at the early stages of manufacturing is critical to achieving reproducible final products. Two key strategies to achieve this goal are optimizing apheresis collection methods and optimizing target cell enrichment. Process optimization of both apheresis and enrichment may differ depending on input parameters. Therefore, there may be no single “right answer” when it comes to process optimization that minimizes variability in all cases. For example, increasing apheresis collection volumes may improve yields in some cases, but worsen contaminating non-target cell frequencies in others. In early phase clinical trials, process flexibility allows for selection from multiple possible pathways based on input parameters. Such variable processing, albeit to achieve reproducible final products, complicates operations and analysis. Furthermore, as processes scale out and approach commercialization, validation of an adaptive manufacturing process is impracticable. Therefore, while generation of reproducible final products may be the overarching challenge facing manufacturers, process standardization as a solution presents operational challenges. A demonstrable understanding of reproducibility in final products is required to evaluate process and product comparability and clearly defined critical quality attributes will drive process standardization in the future.
Cell procurement as a source of variability
Unlike traditional biopharmaceuticals the cellular raw material (CRM) in autologous CGT manufacturing is a donor-derived apheresis product. Apheresis collection is a closed-system, continuous or semi-continuous flow process in which whole blood exits the donor through a sterile single-use tubing set, is separated into components based on centrifugal force, target components are removed and the remainder of the blood is returned to the donor. The entire extracorporeal circuit is short, but the donor’s blood volume may pass through the instrument many times in a single collection. The process is the most efficient method for obtaining a large number of mononuclear cells, however there are considerable limitations to apheresis collection in the areas of yield and purity. Apheresis standardization may be challenging given patient-to-patient variation, but early attempts have been made with some success [2]Allen ES, Stroncek DF, Ren J et al. Autologous lymphapheresis for the production of chimeric antigen receptor T cells. Transfusion. 2017; 57(5): 1133–41. .
The apheresis product obtained from a mononuclear cell collection is a reflection of the circulating frequencies and absolute concentrations of cell types in the donor. This snapshot of cellular components can change drastically based on a variety of donor-related factors. The most striking differences are seen when comparing healthy donors to diseased donors. A number of different immune cell types including but not limited to lymphocytes and monocytes can be sharply decreased in the peripheral blood of patients with hematologic malignancies compared to healthy donors. Further, some hematologic malignancies are marked by high numbers of circulating tumor cells. If these tumor cells are of a specific gravity close to that of lymphocytes, a mononuclear cell collection will also collect these tumor cells. The relative proportion of target immune cells can also be skewed, particularly in diseased donors. For example, monocytes may be overrepresented in the peripheral blood and in the associated apheresis products [3]Nguyen XD, Eichler H, Sucker A et al. Collection of autologous monocytes for dendritic cell vaccination therapy in metastatic melanoma patients. Transfusion. 2002; 42(4): 428–32.. Like some tumor cells, monocytes are of a similar specific gravity to lymphocytes, meaning that an apheresis product from these donors will likely carry excess monocytes, potentially impairing T cell culture. With the advent of automated detection of the white-red cell interface and the ability to adjust collection preference within the mononuclear cell layer, these contaminants can be reduced, but not eliminated entirely [4]Punzel M, Kozlova A, Quade A et al. Evolution of MNC and lymphocyte collection settings employing different Spectra Optia((R)) Leukapheresis systems. Vox Sang. 2017; 112(6): 586–9. In mobilized donor apheresis collection, donor response to mobilization agents can impact the yield of very low frequency cell types such as hematopoietic stem cells. Moreover, the mobilization regimen selected may impact the purity of the collected apheresis product as many commonly used agents also induce mobilization of neutrophils and other contaminating cell types. Standardization of apheresis collection methods will limit the diversity of starting material but not eliminate the challenge of donor variability. In order to achieve reproducible final products, manufacturing platforms need to be responsive to diverse input.
Cell manufacturing platform as a source of variability
A highly adaptive manufacturing process can ensure final product reproducibility with a variable CRM. Process flexibility, as a nimble reaction to the apheresis product, has aided the success of academic and early-phase clinical trials. However, in pursuit of commercial manufacturing success the choose-your-own-adventure style of process flexibility is no longer feasible. A series of sequential enrichment steps are utilized for CGT products to reduce cellular impurities and enrich target cells (Figure 1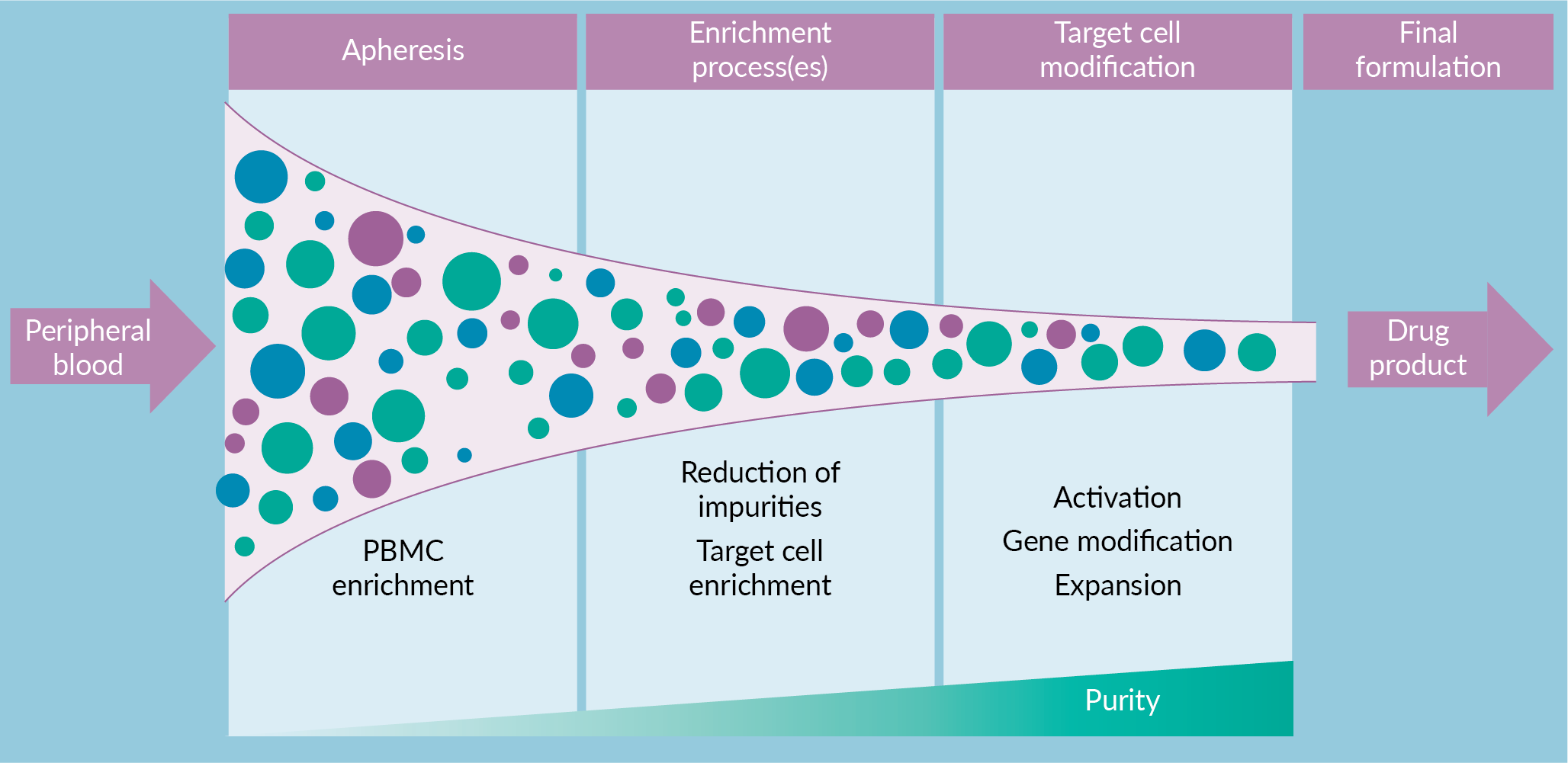
A manufacturing scheme, such as the one described above, that includes sequential application of mass-customizable steps with increasing stringency has the potential to enable final product reproducibility. While this will by definition not lead to a reproducible process, it aims to generate final products that reproducibly meet release specifications. Such an approach puts a special emphasis on these final product specifications. For example, if two separate apheresis products are processed via different enrichments, but both generate a final product with highly pure CAR T-cells, are these products comparable? Evaluating the ability of alternative processes to achieve comparable and reproducible final products is critical. An understanding of process and product impurities will drive continuous process improvement and demonstrate process comparability.
Operational concepts in manufacturing have been adapted to cell therapy biomanufacturing. In more traditional manufacturing settings, mass-customization is a method by which modular elements are standardized but combined in a way to generate a user-customized final product. Manufacturers may produce different sets of components and combine in a customized fashion to meet customer demands. This approach combines efficiencies of mass production with the flexibility to generate reproducible final products through standardized processes. Yet a reactionary approach has negative consequences for cost containment and scalability. Rather, a QbD approach that is responsive to the current challenges will ensure long term success of cell and gene therapeutics.
Tactics to limit variability & improve reproducibility
Variability and uncertainty necessitate process flexibility to achieve the most reproducible final products. However, process flexibility comes at a cost in terms of efficiency, and so it is critical to make judicious use of flexibility when designing a manufacturing platform. In this setting, flexibility refers to the ease of implementing process changes and adopting technological advancements. Both planned and unplanned events can drive the need for change. Planned events, such as a request to manufacture a novel product, is defined by the conscious actions taken by the manufacturer. Unplanned events, on the other hand, occur independent of the manufacturer, yet lead to downstream change as well. The withdrawal of a critical reagent vendor from the market is an example of an unplanned event that requires process flexibility. The ultimate goal of process flexibility is to maximize the likelihood of generating a high quality, reproducible final drug product despite the variability of inputs.
A robust enrichment strategy will pave the way for manufacturing success. A fully automated end-to-end manufacturing solution will ensure process reproducibility and allow for a streamlined approach to demonstrate process control. However, with inherent variability in the starting material there is no one-size-fits-all proposal to ensure reproducibility of the final product. In this case, well-defined in-process controls and appropriate CQAs are required to refine final product specifications. The concept of a modular manufacturing platform is an equally attractive option for achieving process reproducibility but presents a burden on the industry for maintaining a pool of highly trained technologists and may further encumber the limited production capacity of CMOs. Specialized training on an assortment of sophisticated equipment and complex units of operation is already a bottleneck in the CGT industry. Continuous improvement of regulatory guidelines on how to appropriately gauge control of a variable process presents an additional opportunity for progress in this area. When servicing a patient base with high unmet medical needs and clinical urgency, requirements for validation and process performance qualification limit the number of process iterations that can be effectively evaluated. Thus, a reverse engineering approach may be appropriate when there is sufficient clinical data to garner a more complete understanding of potency and efficacy.
In addition, analysis of the optimal cell dose will guide future process developments. Relatively little is understood about appropriate dosing schemes in different patient populations and with different cell types. In the quality vs. quantity debate, purity may in fact be a primary determinant in engineering optimal manufacturing solutions if cell yields are secondary. For example, innovative techniques for target cell enrichment may result in fewer overall cells, but a better performing target population. A recent paper by Radtke et al. highlights a dual enrichment strategy for hematopoietic stem cells using magnetic cell enrichment and sort purification [5]Radtke S, Pande D, Cui M et al. Purification of Human CD34(+)CD90(+) HSCs Reduces Target Cell Population and Improves Lentiviral Transduction for Gene Therapy. Mol. Ther. Methods. Clin. Dev. 2020; 18: 679–91.. Their method enriches cell populations associated with improved engraftment and significantly reduces the need for media and vector consumption. Indeed, the case for quality over quantity was definitively demonstrated in a highly influential paper by Fraietta et al. in which an adult CLL patient achieved long lasting remission through in vivo expansion of a single CAR T-cell clone [6]Fraietta JA, Nobles CL, Sammons MA et al. Melenhorst JJ. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature. 2018; 558(7709): 307–12.. In addition, researchers at the Children’s Hospital of Philadelphia [7]Das RK, Vernau L, Grupp SA, Barrett DM. Naïve T-cell Deficits at Diagnosis and after Chemotherapy Impair Cell Therapy Potential in Pediatric Cancers. Cancer Discov. 2019; 9(4): 492–9. showed that the chemotherapeutic treatment regimen in pediatric oncology patients can influence the cellular composition of apheresis products and may impede successful manufacture of CAR T-cells. Thus, as demonstration of the safety and efficacy of CGT products continues, a paradigm shift in the field to employ these options as first-line therapies may be warranted. Alternatively, apheresis collection and cryopreservation early in the disease course may broaden treatment options to include CGT products if frontline therapies are unsuccessful. Apheresis collection prior to chemotherapeutic regimens may greatly improve manufacturing outcome and aid in standardization of the process.
This challenge also highlights some of the major advantages of allogeneic sourced cell therapies. It certainly is the case that healthy donors have higher circulating frequencies of non-malignant immune cells and no circulating tumor cells. It is also believed that healthy allogeneic donors would exhibit less variability, leading to more consistent apheresis products and ultimately more reproducible manufacturing results. Nonetheless, even in healthy donors, apheresis collection alone may not generate a product with adequate target cell type yields and purities. It may even be wise to consider whether both the apheresis procedure and the downstream enrichment can be jointly adapted to meet specific patient and manufacturing needs.
Reproducible Cell Manufacturing of the Future
Given the complexities associated with vein-to-vein cell manufacturing, it is likely that the control of variability will only be more challenging going forward. As noted above, much of the upfront variability observed is derived from variability in the donor population. It is already known that diverse apheresis products are obtained from seemingly similar patient populations. What has not been well described are accurate and precise predictors of such variability. Many are characterizing parameters in their own patient population, but it follows that donor-derived differences that alter peripheral blood counts will alter apheresis product content. Factors such as the donor’s underlying clinical indication, disease status, prior treatment and recent infection all have the potential to alter apheresis product content in uncontrolled and unexpected ways. As the demand for cell therapies expands to include more patients and patients with different indications, the variability of incoming apheresis products will only increase.
It was highlighted that apheresis and enrichment are sources of variability because they occur early in the process and have significant downstream implications. Yet there are a great many sources of variability not represented here. For example, control and acquisition of raw and ancillary materials is a significant source of process variability in the manufacture of CGT products. The past few years have seen a substantial increase in the availability of GMP-grade reagents along with the number of qualified suppliers. Yet, the vulnerability of supply chain continuity was made wholly apparent in the wake of the COVID-19 pandemic. Assessing the comparability of critical materials and eliminating the use of single-source and sole-source materials remains an area for improvement for manufacturers, suppliers and regulators.
Future cell manufacturing platforms are certain to contend with on-going challenges to reproducibility. Inherently cells, as living organisms, are highly variable. Nonetheless, platform optimization may present ways to limit variability and achieve as reproducible a final product as possible. Specifically, better understanding of optimal autologous apheresis collection timing or use of allogeneic donors may increase the likelihood of a standardized incoming apheresis product. More robust cell enrichment process may allow for standardization if they can efficiently be applied to a variety of inputs. This area of active work promises to improve final product quality, increase manufacturing efficiency, and enable the ability to scale cell and gene therapy products for broad utilization.
References
1. Rader R, Langer E. Fifteen Years of Progress: Biopharmaceutical Industry Survey Results. Pharmaceutical Technology. 2018; 42(7): 56–9. Crossref
2. Allen ES, Stroncek DF, Ren J et al. Autologous lymphapheresis for the production of chimeric antigen receptor T cells. Transfusion. 2017; 57(5): 1133–41. Crossref
3. Nguyen XD, Eichler H, Sucker A et al. Collection of autologous monocytes for dendritic cell vaccination therapy in metastatic melanoma patients. Transfusion. 2002; 42(4): 428–32. Crossref
4. Punzel M, Kozlova A, Quade A et al. Evolution of MNC and lymphocyte collection settings employing different Spectra Optia((R)) Leukapheresis systems. Vox Sang. 2017; 112(6): 586–94. Crossref
5. Radtke S, Pande D, Cui M et al. Purification of Human CD34(+)CD90(+) HSCs Reduces Target Cell Population and Improves Lentiviral Transduction for Gene Therapy. Mol. Ther. Methods. Clin. Dev. 2020; 18: 679–91. Crossref
6. Fraietta JA, Nobles CL, Sammons MA et al. Melenhorst JJ. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature. 2018; 558(7709): 307–12. Crossref
7. Das RK, Vernau L, Grupp SA, Barrett DM. Naïve T-cell Deficits at Diagnosis and after Chemotherapy Impair Cell Therapy Potential in Pediatric Cancers. Cancer Discov. 2019; 9(4): 492–9. Crossref
Affiliations
Anne Lamontagne MS
Rocket Pharmaceuticals
Andrew Fesnak MD
Department of Pathology and Laboratory Medicine, Perelman School of Medicine at the University of Pennsylvania
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2020 Lamontagne A & Fesnak A. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Aug 24 2020; Revised manuscript received: Oct 12 2020; Publication date: Nov 4 2020.
