What to expect in 2021: seven cell and gene therapy commercialization trends
Cell & Gene Therapy Insights 2020; 6(11), 1539–1547
10.18609/cgti.2020.169
The year 2020 has been noteworthy for cell and gene therapies (CGTs), which conservatively account for 12% of the pharmaceutical industry’s clinical pipeline and 16% of its preclinical pipeline. Although COVID-19 has slowed some clinical studies and key research and development activities, investments and financing in the regenerative therapy market have remained robust – and more than 1,000 therapeutic developers remain active in the space worldwide [1].
As the Alliance for Regenerative Medicine (ARM) shared in its 2020 mid-year Global Regenerative Medicine & Advanced Therapy Sector Report, despite unprecedented global challenges caused by COVID-19, the CGT market attracted $10.7 billion in capital globally in just the first 6 months of 2020, exceeding the total amount raised in all of 2019.
Further buoying continued growth is recent research that demonstrated the cost-saving potential of CGTs in the treatment of rare blood diseases including multiple myeloma, hemophilia A and sickle cell disease. The report found that within these diagnoses alone, CGTs have the potential to reduce total disease costs by 18 to 30%, representing an aggregate cost savings of more than $33 billion over 10 years [1]Alliance for Regenerative Medicine. ARM Global Regenerative & Advanced Therapy Medicine Sector Report: H1 2020 – Innovation in the time of COVID-19. Washington, D.C. Alliance for Regenerative Medicine. 2020; 6, 13, 19: https://alliancerm.org/sector-report/h1-2020-report/Alliance for Regenerative Medicine. ARM Global Regenerative & Advanced Therapy Medicine Sector Report: H1 2020 – Innovation in the time of COVID-19. Washington, D.C. Alliance for Regenerative Medicine. 2020; 6, 13, 19: https://alliancerm.org/sector-report/h1-2020-report/Alliance for Regenerative Medicine. ARM Global Regenerative & Advanced Therapy Medicine Sector Report: H1 2020 – Innovation in the time of COVID-19. Washington, D.C. Alliance for Regenerative Medicine. 2020; 6, 13, 19: https://alliancerm.org/sector-report/h1-2020-report/Alliance for Regenerative Medicine. ARM Global Regenerative & Advanced Therapy Medicine Sector Report: H1 2020 – Innovation in the time of COVID-19. Washington, D.C. Alliance for Regenerative Medicine. 2020; 6, 13, 19: https://alliancerm.org/sector-report/h1-2020-report/. While innovative financing models – such as subscription models, payment-over-time, and value-based payments are needed to help offset the potentially high up-front costs of these therapies, the longer-term cost-saving potential of CGTs will be another factor that bolsters growth.
As healthy as financing expectations are for the CGT sector, the reality is that this is an emerging market, built on cutting edge science, and new solutions are needed for a number of practical business and operational challenges in order to realize its full growth potential. These new solutions will optimize the CGT value chain, or the full range of activities that make a product valuable. The drug product value chain starts at product development and continues through patient identification, production, marketing, treatment, and post-treatment activities. It includes the supply chain and expands upon it vertically and horizontally as the different supply chain components are incorporated and combined to create value.
Working together with other stakeholders to define the operational processes and systems for new standards of care gives us an informed and broad perspective on what’s next for CGT and the value chain. Here are seven commercialization trends that we’ve identified with one of those collaborators, Cardinal Health Specialty Solutions, that are likely to shape the CGT market, along with practical steps to accelerate not just approval, but adoption of these innovative therapies.
1. The effects of COVID-19 will continue to ripple across CGT
While investment in CGTs is likely to continue, there is no denying that timelines for clinical trials and approvals will continue to be affected by COVID-19. For example, Food and Drug Administration (FDA) officials have expressed concern about the impact that COVID-19 will have on clinical trials, acknowledging that the pandemic has caused considerable challenges for some CGT studies, particularly in terms of reaching their clinical endpoints [2]U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Center for Devices and Radiological Health (CDRH), Oncology Center of Excellence (OCE) and Office of Good Clinical Practice (OGCP). FDA Guidance on Conduct of Clinical Trials of Medical Products during COVID-19 Public Health Emergency: Guidance for Industry, Investigators, and Institutional Review Boards. Silver Spring, MD. The FDA. March 2020, updated September 21, 2020: https://www.fda.gov/media/136238/download.
At ARM’s virtual Meeting on the Mesa, held in October 2020, Peter Marks, MD, PhD, Director, Center for Biologics Evaluation and Research, US Food and Drug Administration (FDA), acknowledged that the pandemic has disrupted global harmonization efforts around CGTs – and that it has forced many FDA reviewers to shift priorities. Even reviewers such as Dr Marks, who before COVID-19, would have spent about 75% of his time on CGTs, are now spending the majority of their time on COVID-related activities (Figure 1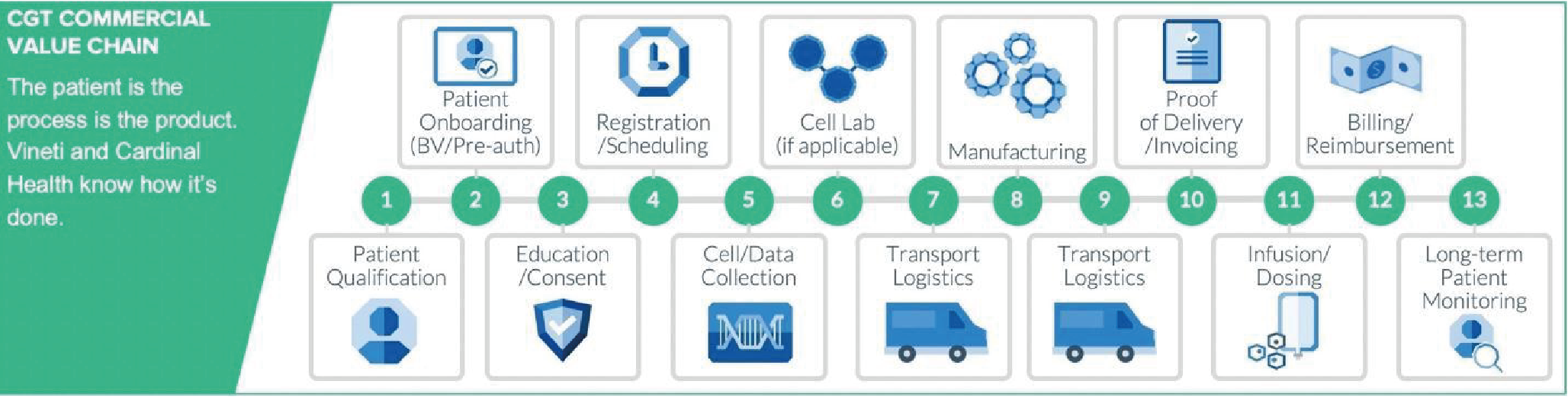
2. CGTs used to treat COVID-19 and its long-term complications may expand the reach of these cutting-edge therapies
From a clinical perspective, ARM also reports that several academic research centers and therapeutic developers are investigating the application of regenerative medicine technologies to treat COVID-19 in the short-term and address related, long-term complications [1]Alliance for Regenerative Medicine. ARM Global Regenerative & Advanced Therapy Medicine Sector Report: H1 2020 – Innovation in the time of COVID-19. Washington, D.C. Alliance for Regenerative Medicine. 2020; 6, 13, 19: https://alliancerm.org/sector-report/h1-2020-report/Alliance for Regenerative Medicine. ARM Global Regenerative & Advanced Therapy Medicine Sector Report: H1 2020 – Innovation in the time of COVID-19. Washington, D.C. Alliance for Regenerative Medicine. 2020; 6, 13, 19: https://alliancerm.org/sector-report/h1-2020-report/Alliance for Regenerative Medicine. ARM Global Regenerative & Advanced Therapy Medicine Sector Report: H1 2020 – Innovation in the time of COVID-19. Washington, D.C. Alliance for Regenerative Medicine. 2020; 6, 13, 19: https://alliancerm.org/sector-report/h1-2020-report/Alliance for Regenerative Medicine. ARM Global Regenerative & Advanced Therapy Medicine Sector Report: H1 2020 – Innovation in the time of COVID-19. Washington, D.C. Alliance for Regenerative Medicine. 2020; 6, 13, 19: https://alliancerm.org/sector-report/h1-2020-report/.
By mid-year 2020, at least 11 clinical trials using regenerative medicine and advanced therapy technologies in the treatment of COVID-19 were already in motion, with an additional 25 programs in preclinical development [1]Alliance for Regenerative Medicine. ARM Global Regenerative & Advanced Therapy Medicine Sector Report: H1 2020 – Innovation in the time of COVID-19. Washington, D.C. Alliance for Regenerative Medicine. 2020; 6, 13, 19: https://alliancerm.org/sector-report/h1-2020-report/Alliance for Regenerative Medicine. ARM Global Regenerative & Advanced Therapy Medicine Sector Report: H1 2020 – Innovation in the time of COVID-19. Washington, D.C. Alliance for Regenerative Medicine. 2020; 6, 13, 19: https://alliancerm.org/sector-report/h1-2020-report/Alliance for Regenerative Medicine. ARM Global Regenerative & Advanced Therapy Medicine Sector Report: H1 2020 – Innovation in the time of COVID-19. Washington, D.C. Alliance for Regenerative Medicine. 2020; 6, 13, 19: https://alliancerm.org/sector-report/h1-2020-report/Alliance for Regenerative Medicine. ARM Global Regenerative & Advanced Therapy Medicine Sector Report: H1 2020 – Innovation in the time of COVID-19. Washington, D.C. Alliance for Regenerative Medicine. 2020; 6, 13, 19: https://alliancerm.org/sector-report/h1-2020-report/. Approaches include therapies intended to promote immune response and manage inflammatory responses in patients treated for COVID-19, as well as repairing longer-term tissue damage caused by the disease. Many developers are utilizing mesenchymal stem cells (MSCs), and other stromal cells that have the ability to impact inflammation pathways, to treat Acute Respiratory Distress Syndrome (ARDS), a severe complication of COVID-19.
Lessons learned from complications of CAR-T cell therapies may also help inform treatment of COVID-19. Both CAR-T cell therapies and COVID-19 infections sometimes result in cytokine release syndrome (CRS) as a complication. According to research leaders, treatments and learnings used to address this rapid, sometimes life-threatening, immune response in CAR-T patients may also provide insights for COVID-19 patients [3]McCullough, Marie. Tackling coronavirus is the next challenge for Penn cancer pioneer. The Philadelphia Inquirer. April 16, 2020: https://www.inquirer.com/health/coronavirus/coronavirus-carl-june-t-cell-cancer-therapy-offers-lessons-20200416.html?cid=Philly.com+Twitter&utm_campaign=Philly.com+Twitter+Account&utm_medium=social&utm_source=t.co.
3. CGT Chemistry, Manufacturing and Controls (CMC) guidance will be applied with even broader rigor
Given the personalized, patient-specific nature of many CGT drug products, CMC carries increased importance during regulatory reviews. According to remarks by former FDA Commissioner Scott Gottlieb, MD, the traditional paradigm “where 80% of the review is focused on the clinical portion of that process, and maybe 20% is focused on the product issues...is almost completely inverted when it comes to cell and gene therapy [4][cited 2020 Oct 30]:https://www.fda.gov/news-events/speeches-fda-officials/remarks-alliance-regenerative-medicines-annual-board-meeting-05222018.” (Figure 2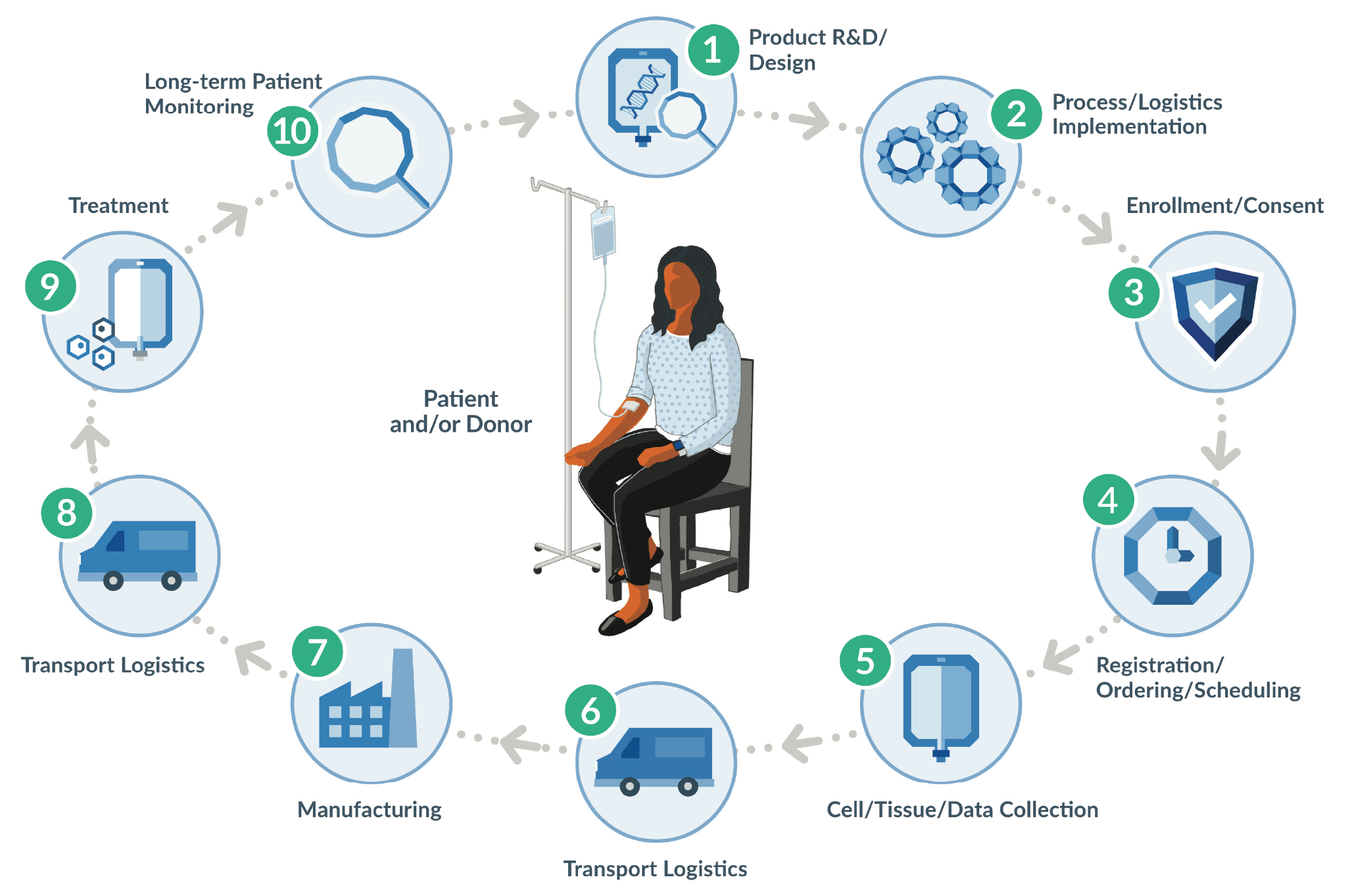
In 2020, several cell therapy Biologics License Applications (BLAs) were slowed due to CMC questions, primarily due to the FDA requesting further detail. With more than 1,000 CGT developers currently pursuing clinical trials and with many exploring the use of these therapies to address much broader health indications, such as solid tumors, cardiac disorders, or inflammatory conditions, the FDA and other regulatory bodies are expected to extend rigor broadly on CMC processes.
In early 2020, the agency issued a CMC guidance for advanced therapies, and the agency’s actions over the course of the year indicated that regulators will apply the guidance thoroughly [5]US Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research. Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs): Guidance for Industry. Silver Spring, MD. The FDA. January 2020: https://www.fda.gov/media/113760/download. This increased focus will make it all the more important for drug product developers to leverage innovations such as digital workflow management and supply chain orchestration technology to accurately capture, report, and analyze all clinical, patient handling, case management, and supply chain data. Enterprise-grade, purpose-built supply chain and data management platforms are key to collecting and reporting detailed, rigorous CMC data.
4. Leadership of independent, cross-industry organizations, such as the Standards Coordinating Body for Regenerative Medicine, will continue to grow in importance
In order to expedite not just the approval, but the use of CGTs, developers will also quickly need to distinguish between areas of competitive advantage and opportunities to standardize for the sake of speed and progress.
Because the science behind CGTs is relatively new, the field of regenerative medicine faces challenges that are common to many emerging industries – including fragmentation of knowledge, insufficient communication and coordination, and unpredictable innovation. It can take decades for new industries to establish standards that address these issues and accelerate innovation.
Here, however, the lives of hundreds of thousands – and potentially millions – of patients depend on all stakeholders, including researchers, product developers, raw material providers, government and regulatory agencies, clinicians, and healthcare professionals, to dramatically expedite the pace of standards development.
Leadership from independent, objective, non-profit organizations – specifically the Standards Coordinating Body for Regenerative Medicine – will play an increasingly critical role in accelerating the development and use of standards that improve the safety and quality of regenerative medicines, with the ultimate goal of reducing costs, complexity, and risk to serve more patients.
In-process labeling for CGT drug products is one current, important area of standardization, as it represents real-world application of Chain of Identity (COI). This required patient safety component, defined in the diagram below, is essential to ensuring that the right patient receives the right CGT product. The Standards Coordinating Body is currently conducting an industry-wide working group to drive new and updated standards for in-process labeling and related COI issues in cell collection procedures. To learn more and get involved, please visit the Standards Coordinating Body website. To learn more about in-process labeling and label printing for CGTs, please see this peer-reviewed article in Cell and Gene Therapy Insights [6]Hagen H, Suchet C. Small labels, big challenges: solutions for advanced therapy labeling. Cell Gene Ther. Insights 2020; 6(8), 1183–95. (Figure 3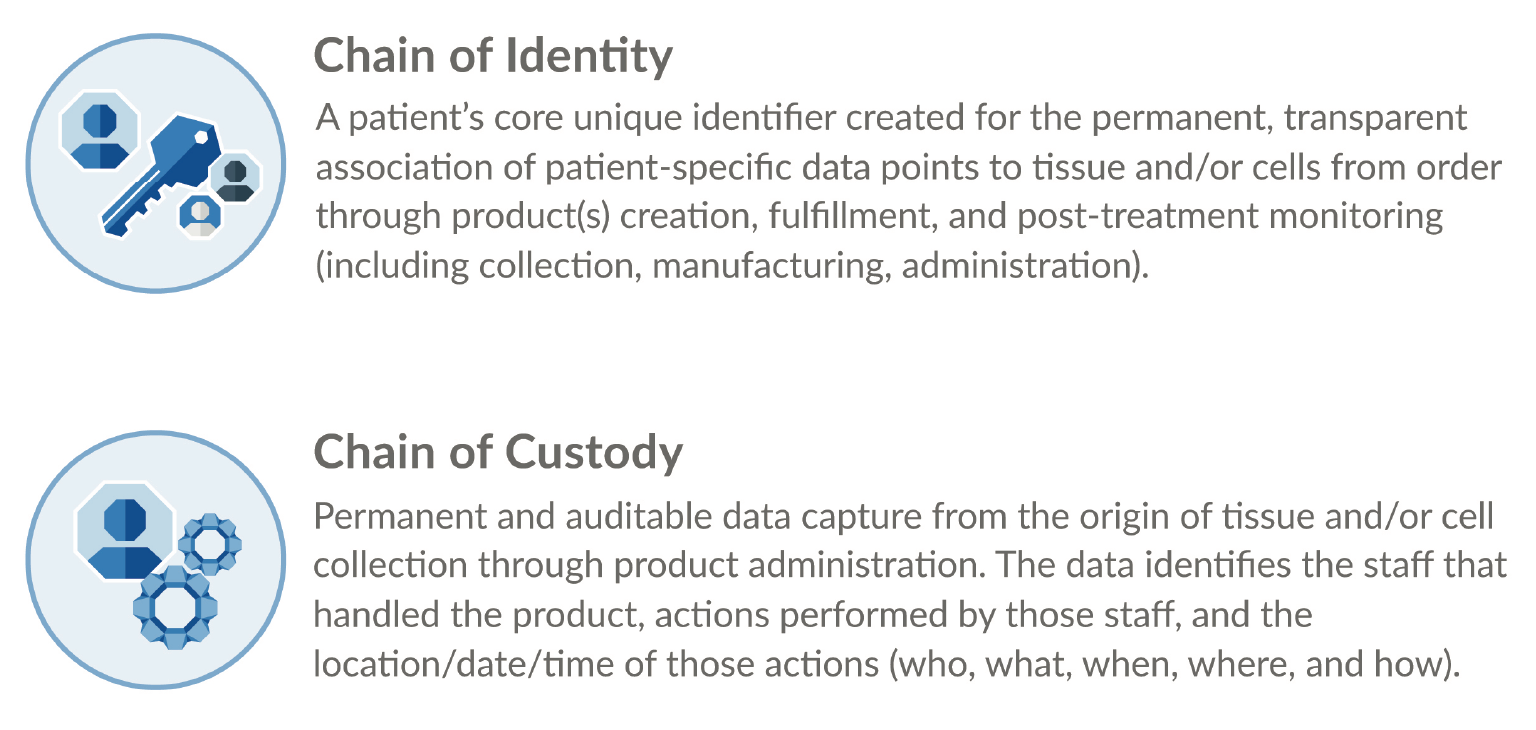
5. More research with sites of care will be needed to identify best practices and provide education to care teams
More than 100 hospitals across the USA are administering the currently available CGTs, according to insights from healthcare centers and manufacturers. Each site of care is determining how best to coordinate patient care. While this exploration is likely to drive innovation and invaluable learnings, identifying the real-world approaches and best practices that are having the most positive impact on patient outcomes will also be key to driving greater adoption.
Developers can support this process by tracking key metrics at certified administration facilities – such as the number of CGT patients served, the number of days it takes each facility to coordinate care from the point of apheresis to infusion, the average length of time it takes at each facility to successfully navigate the prior approval process, and other key metrics. Of course, developers are also well advised to track patient outcomes over time, to begin to determine which practices are having optimal impact.
This kind of research can be parlayed into education and best practice guidelines to help hospitals, and eventually, community providers, understand:
The role that patient navigators can play in improving patient satisfaction and patient outcomes. This includes the development of models for aligning and providing the clinical, financial, and social support that patients need throughout their care journey;
Various staffing models for delivering optimal care to patients receiving CGTs, based on the size of the institution and number of regenerative medicine patients the facility has the potential to serve. This includes guidance on how best to address specialized care needs that require expertise from intensive care, neurology, and other specialists;
Models for expediting all phases of the care coordination process, from benefits investigation to apheresis to infusion;
Key post-acute care issues that impact patient outcomes and models for helping providers avoid, or more quickly address, those challenges.
6. We’ll see even more demand for truly industrialized, rigorous digital capabilities that simplify CGTs
The seamless and efficient orchestration of CGT order management, product manufacturing, and delivery requires close coordination among all value chain stakeholders. Sophisticated digital tools provide the collaboration that simplifies the process of ordering and tracking the delivery of each patient-specific dose as it moves through the value chain.
Continued feedback from healthcare providers (HCPs) indicates that most facilities currently administering CGTs find they are being encumbered by too many disparate workflows and systems. HCPs are allocating far too much valuable staff time to learning, managing, and utilizing many different product ordering and tracking portals and processes – which are different for each CGT product. In some cases, healthcare facilities are assigning multiple staff members to manage the ordering and reimbursement process for CAR-T medications – in addition to the work these teams are already providing on patient-facing apheresis, administration, and patient follow up processes that are associated with CGTs.
These disparate, non-standardized systems will not be practical as dozens of new CGTs become commercialized and thousands more move through clinical trials over the next five years. Healthcare providers will increasingly demand that the ordering and product tracking processes for these products be as intuitive, consistent, and user-friendly as the processes they follow for traditional treatments.
That’s one reason why Cardinal Health Specialty Solutions recently established a collaboration with Vineti to create an integrated digital platform that enables developers to provide sites of care with a more user-friendly, intuitive, and consistent way to order personalized medicines, and track those therapies as they travel through the CGT value chain [7]Vineti, Inc. Cardinal Health and Vineti. That value chain transparency – knowing exactly where each patient-specific dose is at all times – is particularly critical to healthcare providers who are scheduling therapy administration for extremely fragile patients.
By developing digital technology systems to make the process of ordering and tracking personalized medicines easier, less time consuming, and more consistent and predictable, industry players such as Cardinal Health Specialty Solutions and Vineti also aim to expand access to these cutting edge therapies for thousands more patients over time, including those served by community hospitals as safety protocols permit.
7. Developers will increasingly differentiate their therapies by introducing or strengthening patient hub services
While standardization is still needed in order to expedite the adoption of CGTs in real world settings, wrap-around patient hub services are an area where developers will increasingly seek to differentiate their therapies.
Some administration sites are already innovating in this area and have patient navigators whose sole purpose is to serve as a single point of contact for coordinating the care of CGT patients. However, not all large hospitals have the resources to invest in patient navigators, and as CGT developers work to expand access to their therapies among more patients, it’s even less likely that community hospitals will have the resources to invest in patient care navigators who are proving to be so crucial to supporting positive patient outcomes.
In cases where these navigator roles don’t exist, developers will differentiate their therapies and improve the journey for these often-fragile patients and their caregivers through patient hubs.
Patient hubs can help facilitate and simplify everything from benefits investigation to intake processes and emotional support. They can also deliver critical support services that tackle barriers to care, such as lack of access to transportation and lodging, while serving as a means for developers to collect the essential information needed to monitor and track patient outcomes. With much of the access path to CGTs being non-standard, understanding the depth of experience of hub partners in this space can help ensure developers don’t miss a step on their own path to market.
Developers and investors have collectively directed billions of dollars toward the development and commercialization of CGTs. However, the commercial success of these therapies – and the degree to which these therapies are adopted broadly on behalf of the patients who so desperately need them – will depend heavily on the ability of all stakeholders in the value chain to work together to help care providers and patients alike. Utilizing experienced operational partners and leveraging enterprise-grade purpose-built digital systems will be essential to expanding CGTs in 2021 and beyond, simplifying complex workflows and driving widespread adoption.
References
1. Alliance for Regenerative Medicine. ARM Global Regenerative & Advanced Therapy Medicine Sector Report: H1 2020 – Innovation in the time of COVID-19. Washington, D.C. Alliance for Regenerative Medicine. 2020; 6, 13, 19: view here. Crossref
2. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Center for Devices and Radiological Health (CDRH), Oncology Center of Excellence (OCE) and Office of Good Clinical Practice (OGCP). FDA Guidance on Conduct of Clinical Trials of Medical Products during COVID-19 Public Health Emergency: Guidance for Industry, Investigators, and Institutional Review Boards. Silver Spring, MD. The FDA. March 2020, updated September 21, 2020: view here. Crossref
3. McCullough, Marie. Tackling coronavirus is the next challenge for Penn cancer pioneer. The Philadelphia Inquirer. April 16, 2020: view here. Crossref
4. [cited 2020 Oct 30]:https://www.fda.gov/news-events/speeches-fda-officials/remarks-alliance-regenerative-medicines-annual-board-meeting-05222018 Crossref
5. US Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research. Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs): Guidance for Industry. Silver Spring, MD. The FDA. January 2020: view here. Crossref
6. Hagen H, Suchet C. Small labels, big challenges: solutions for advanced therapy labeling. Cell Gene Ther. Insights 2020; 6(8), 1183–95: view here. Crossref
7. Vineti, Inc. Cardinal Health and Vineti Crossref
Affiliations
Heidi Hagen
Co-founder and Advisor, Vineti
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: The author is Co-founder and Advisor at Vineti Inc., the author declares that that they have no other conflicts of interest.
Funding declaration: The author received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2020 Vineti Inc. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited.
Revised manuscript received: Nov 18 2020; Publication date: Dec 14 2020.

.png)