Managing starting material stability to maximize manufacturing flexibility and downstream efficiency
Cell & Gene Therapy Insights 2019; 5(2), 303-314.
10.18609/cgti.2019.033
The cell and gene therapy industry is filled with excitement and promise as recent clinical and commercial successes make headlines worldwide. New therapies are entering clinical trials like never before [1]. With this growth comes underlying challenges, some of which are obvious and well documented, such as scale-up manufacturing and the need for cost of goods reduction [2] – but others are perhaps not currently given the amount of attention they deserve or require. Two such challenges are the increasing demand for quality starting materials and the logistical issues in getting those products, typically shipped fresh from the clinic or donor processing center to the manufacturing facility [1,3,4]. There are many time-sensitive elements within the cell and gene therapy supply chain and these present critical challenges. This article will explore some specific examples and look at how the industry can address them by improving quality and consistency of starting materials and reducing some of the existing bottlenecks which may impact downstream manufacturing and therapeutic success.
Everything begins with the starting material
The value of starting material is vital to the industry: as the old saying goes, ‘garbage in equals garbage out’ and if you begin with poor starting material, you’re likely to end up with an inconsistent process and a poor outcome. This is particularly true for cell therapy, whether autologous (i.e., the patient’s own cells) or allogeneic (i.e., generic cells from a donor). In both cases, the starting material represents the most variable part of the process, whether it is in early stage development or in the clinical/commercial manufacturing setting [1].
Since donors and patients are all unique, there is an inherent variability in the cells derived from them. Since there is a finite supply, it is even more important to maximize not only the quality, but also the availability of that material.
After source material collection, we move into processing and packing immediately for shipping to the manufacturing site where further processing and isolation, selection and expansion and purification steps occur. This is followed by a degree of biopreservation to enable shipping to the patient for administration (Figure 1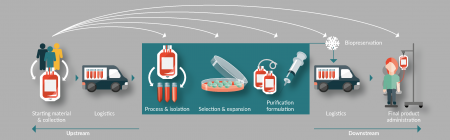
Historically, the cell therapy industry has invested a great deal of time and capital into logistical issues and much of this has focused primarily on biopreservation of the final product and maintaining the cold chain [4,5]. Solution providers have entered the market specializing in the transportation of cell therapy products, but the challenge we are now experiencing is the requirement for that starting material to be both available in sufficient quantity and of the highest quality to ensure consistency downstream. Attention is therefore starting to shift to logistics and biopreservation at the front end of the supply chain.
Impacting stability
Several elements impact the stability of the starting material, not the least of which is time. Figure 2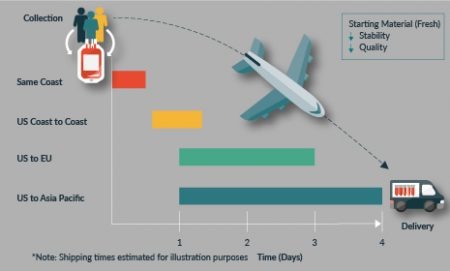
What this ultimately means is that the cells to a varying degree will begin to die and the quality of the material will reduce. This is reflected in parameters such as cell viability, number, and critical quality attributes, which can affect downstream processing.
One aspect that is often overlooked upfront by developers and manufacturers is the availability of resources for receiving and processing the starting material. Certainly, manufacturing that relies upon just-in-time shipment and delivery of the material is inherently inflexible. For example, if one expects a product to be received at 9 a.m. one morning, but it’s delayed until the afternoon, any reserved manufacturing space and personnel will have been standing idle. This is going to ultimately impact the cost of goods and also the shelf-life limits of both your materials and the final product.
Starting materials are commonly shipped fresh at ambient temperatures or hypothermic in autologous plasma at 4°C. Similar to cell therapy final products however, given limits to their shelf-life and stability, high demand for access to products, and the risk of diminishing quality and consistency, other options should certainly be considered [5]. For final product stability, cryopreservation has subsequently become a more standard approach to ensure stability and consistency are maintained (e.g., Novartis’ Kymriah and Kite’s Yescarta).
Given the complexity, not all products are going to be cryopreserved. Other options to consider may be hypothermic in specialized storage mediums – for example, HypoThermosol (Biolife Solutions) – which can potentially extend shelf-life and stability and offer the required flexibility to support product requirements.
Benefits of cryopreservation for extending starting material stability & shelf-life
Historical studies have supported the shift away from shipping cell-based products at room temperature to using hypothermic conditions to extend and preserve shelf-life and quality [6,7]. Data has demonstrated that hypothermic storage can improve the stability and extend shelf-life of starting materials like bone marrow and peripheral blood stem cells. The study by Kao et al. also noted the decline in cell function over time may be more significant than observed with cell viability [6]. However, while hypothermic conditions offer improved stability of starting materials, significant loss in both cell viability and function can still occur over the initial 24–48 hours post collection. Methods to preserve the starting material quality at the point of collection and subsequently extend shelf-life are important. Options to consider may be the inclusion of an optimised storage medium (e.g., HypoThermosol) or cryopreservation.
To develop this further, collaborative studies were performed to evaluate alternative methods for extending shelf-life and improving stability of donor-derived leukapheresis products as a means of ultimately providing enhanced quality and flexibility for the downstream processing and manufacturing consistency. For both studies, three unique donor leukapheresis collections (two separate studies) were performed (HemaCare Corp, Northridge, CA). Following collection, the products were processed and split onsite with half of the product placed in HypoThermosol (BioLife Solutions), while the other half was cryopreserved in CryoStor CS10 (BioLife Solutions). Both sets of products were then shipped with the downstream processing being performed at the respective collaborating facilities.
In the first study, the clinical research facility received and processed the incoming leukapheresis material 24 hours post-collection and evaluated the product for recovery and viability of white blood cell (WBC) and CD14+ cells. While some donor to donor product variability was observed, viability and recovery of WBC post hypothermic storage (24 hours) in HypoThermosol or cryopreservation were similar (Figure 3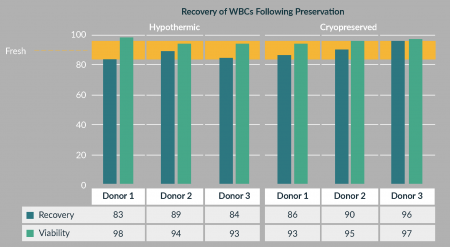
In addition to WBC, the study also analyzed total CD14+ cell viability, recovery and function. Similar to the WBC results, total viable CD14+ cell recovery ranged was 78% to 94% (average of 89%) and was similar between preservation conditions. CD14+ cell function was maintained and similar to preconditioned controls. Collectively, the study demonstrated that cryopreservation and hypothermic storage (using an optimized media) are both viable options to extend stability and shelf-life and to maintain quality of the starting material.
For the second study, the product stability was evaluated under four different conditions in order to understand the impact of the process timing for both hypothermic and cryopreserved starting material. To evaluate this, the leukapheresis was collected and initially prepared at HemaCare (time zero) and then shipped overnight to Hitachi Chemical Advanced Therapeutics Solutions (HCATS), where performance was evaluated by viable cell recovery and a naivety panel (CD45RA and CCR7) over a total of 120 hours. Four different conditions were evaluated for this study:
- Unmanipulated leukapheresis (held at 2–8°C)
- Ratio of 1:1 leukapheresis to CS10 (cryopreserved)
- Ratio of 1:1 leukapheresis to HypoThermosol (held at 2–8°C)
- Ratio of 1:1:2 leukapheresis to HypoThermosol to CS10 (cryopreserved)
The results of this study can be seen in Figure 4 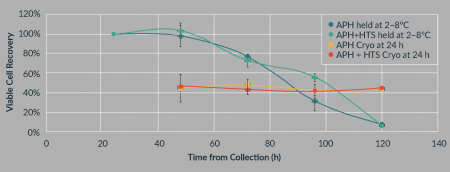
Figure 4, shows the naivety of the CD3 cell population, which represents a sub-population of the CD3+ cells. In the leukapheresis held at 2–8°C, the population naivete is ~60% after 24 hours and 50–60% after 96 hours. However, in the cryopreserved leukapheresis the naivete has been reduced to ~30%, a dramatic decrease in this quality marker, indicating that cryopreservation may have a negative impact on product quality. That said, if starting material stability is required for a period exceeding 96 hours, cryopreservation is a viable method to ensure stability and allows for simplified scheduling in manufacturing. Production demand can be level-loaded by holding product for specific manufacturing slots, rather than requiring on-demand manufacture.
Considering the data and study results collectively, it is important to understand your downstream processing or manufacturing requirements of the starting material to apply the most effective biopreservation platform. Working with fresh material is ideal, but stability in the form of cell viability and function begin to decline immediately following collection (Figure 5
Historical data from the literature has demonstrated that hypothermic conditions can offer some added stability and shelf-life, but certainly not without its limitations. Cryopreservation is known to extend shelf-life and offer long-term stability for cell-based products [1]. Studies described herein have also demonstrated the efficacy of inclusion of cryopreservation for starting materials. Traditionally, cryopreservation has been performed post-collection at the manufacturing site (e.g., CDMO such as HCATS will often receive the starting material 24 hours after collection) prior to performing extended processing steps. The issue here, as described in the aforementioned collaborative study, is the stability and quality of the starting material is already compromised by the time it is processed and cryopreserved. Alternatively, some collection facilities (e.g., HemaCare GMP collection and processing facility) are able to perform cryopreservation at the point of collection (Figure 6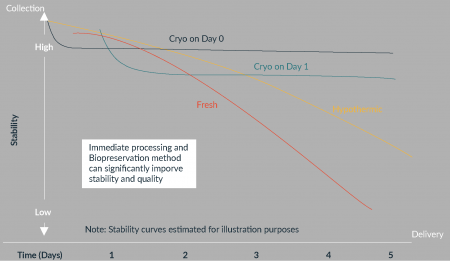
The CDMO perspective
Moving down the chain from collection into downstream processing, it becomes quite clear how important the starting material collection is as the first step of the cell journey. If this is where we are going to begin, we need to ensure premium quality and consistency. Any decline in these standards at this early stage of processing will have a significant negative impact on the complexities we encounter at the manufacturing site.
There is of course a big difference between patient-specific and off-the-shelf therapies when it comes to starting material. With the celebrated emergence of patient-specific CAR T therapies, there’s been more of a focus on the autologous area, but there continues to be a big question mark about how we manufacture and supply this novel product. Therefore it’s imperative that vendors, developers, regulators and manufacturers continue to work through the challenges in a collaborative way to ensure that optimum solutions are created.
Collection is a controllable step. It is something that has been done for years, going back to the first blood transfusions and hematopoietic stem cell transplants. We have seen the beginnings of automation and documented procedures to improve consistency, but there still remains a considerable manual, operator-specific art to collection. Site-to-site variation is also considerable, although this is a fact which can be missed until later stage clinical trials: at early development and even Phase 1 clinical stages trials, oftentimes just a single collection site is used. It is vital to tackle this challenge upfront, knowing that a single collection site will not be feasible for later stage and commercial processing. For example, data from the last 3 months for one of the processes performed at HCATS shows low collections of 7 billion total nucleated cells up to highs of 145 billion – a 20-fold variation.
There is a need to have a funnel approach to processing at a manufacturing site, where all starting materials come in and a more consistent product comes out at the end. The challenge of the funnel is that the more complexity and variability that we start with, the more variable the manufacturing process becomes considerably increasing both cost and time. Developed industries, such as the automotive industry, are now moving beyond tolerances of six sigma. For the cell and gene therapy industry today, that seems a near-impossible stretch, yet the more mitigation of variation that can be achieved, the more rapidly we can grow to ultimately increase patient access.
So how can we improve the consistency of starting material? Removing any living entity from its natural habitat induces a certain amount of stress and this is no different for cells as they are moved from an in vivo to an in vitro environment. This issue is also compounded over time, so it is important to determine a method that either allows for rapid manufacturing, such as bedside operations (although this seems to be a future vision that is some way off commercial use) or one that allows for preservation of the cells. As we transition into global manufacturing, clinical site-to-manufacturing-site-to-patient distances are likely to increase, requiring longer stability times for both starting and final products. As these logistical timeframes increase, so too does the likelihood of delays. As discussed previously, this has the knock-on effect of not only starting material deterioration, but also inefficient scheduling of resources, personnel, and clean rooms. Currently, it is common that an apheresis is delayed by half a day, which means the personnel is delayed by half a day. If the employees can only work 8 hours a day, this means they can only process for 4 hours so a new team must be on standby, thus increasing both cost of labor and the number of staff who require specific training. On top of this, perhaps the next process coming into the cleanroom will also be delayed as part of the knock-on effect. The net effect of all this is increased complexity, increased cost, increased deviations, and further variability in manufacturing process time.
A further supply chain challenge is presented by the need to accommodate the complete bill of materials required to execute a variable process. Taking the earlier example of a 20-fold increase to 145 billion cells, this will involve different container sizes and centrifuge tubes to accommodate the variance. All of the potential containers will need to be kept on standby ready to bring into the cleanroom, or to have in the cleanroom ready to utilize. The batch record will also need to have multiple options for an operator to pick from given the starting material characteristics, further increasing the manufacturing complexity.
There is one everyone is talking about – the desire to automate in order to deliver greater consistency. Automation of a variable process is vastly expensive, challenging and potentially something that’s starting to hold the field back. There’s a lot of automation out there, a lot of ideas, but being able to implement it in a cost-effective and quality-effective manner is extremely hard for variable processes. The majority of automation in this industry does not offer the flexibility that is required in today’s clinical setting.
Conclusion
While the concept of fresh cells is appealing, it doesn’t necessarily mean it will ultimately be the best option, given the potential for delays along the length of the supply chain. Both autologous and allogeneic material require transport post-collection for downstream manufacturing, and extending the shelf-life of the starting material is going to be a necessity for the industry as it maintains quality but also improves flexibility and maximizes downstream manufacturing capacity, to help to achieve the best possible final product.
In summary, as the quality or consistency of a starting material decreases, the complexity and therefore the cost of manufacturing increases.
There are many solutions out there that can start minimizing starting material variation. And working with the key partners having this experience is vital to ensuring product success.
Today, cell therapy manufacturers continue to deal with a certain degree of variation making it difficult and costly, which increases deviations as the variation and complexity increases. As the industry and regulatory landscape continue to evolve, it’s vital that as an industry, we take a lifecycle approach to risk management – a periodic review, phase-gated approach to ensure that we mitigate as much risk as possible all the way from discovery through to commercialization.
It’s vital that both manufacturers, and developers stay ahead of this variability issue and control it as effectively as possible. Let’s work together to ensure we can get there, and make sure that if we’re demanding this consistent quality from our vendors, that really translates all the way through the manufacturing process to the success of the product and success for the patient.
Financial & competing interests disclosure
Dominic Clarke is an employee of HemaCare Corporation. He has no financial conflict with the subject matter or materials discussed in the manuscript. David Smith is an employee of Hitachi Chemical Advanced Therapeutics Solutions He has no financial conflict with the subject matter or materials discussed in the manuscript. This includes, consultancies, honoraria, stock options or ownership, expert testimony, grants or patents received or pending, or royalties.This article has been written based on a webinar presentation by Dr Clarke and Dr Smith for Cell and Gene Therapy Insights, the On Demand recording of which can be accessed here free of charge.
References
1. Juliano L, Eastwood G, Berard T, Mathew AJ. The Importance of Collection, Processing and Biopreservation Best Practices in Determining CAR-T Starting Material Quality. Cell Gene Ther. Insights 2018; 4(4): 327–36. CrossRef
2. Challener C. Cell and Gene Therapies Face Manufacturing Challenges. BioPharm International 2017; 30(1).
3. ISCT 2018 Commercialization Signature Series. Cell Gene Ther. Insights 2018; 437-56.
4. Ellison S, McCoy R, Bell M, Frend K, Ward S. Logistics by Design: A framework for advanced therapy developers to create optimal Logistics Platforms. Cell Gene Ther. Insights 2018; 4(10): 1019–40. CrossRef
5. Meacle F et al. Key considerations of cell and gene therapy cold chain logistics. Cell Gene Ther. Insights 2016; 2(2): 223–36. CrossRef
6. Antonena V, Garvin F, Webb M, Sartor M, Bradstock KF, Gottlieb D. Fresh PBSC harvests, but not BM, show temperature-related loss of CD34 viability during storage and transport. Cytotherapy 2006; 8(2): 158–65. CrossRef
7. Kao GS, Kim HT, Daley H et al. Validation of short-term handling and storage conditions for marrow and peripheral blood stem cell products. Transfusion 2011; 51(1): 137–47. CrossRef
Affiliations
Dominic Clarke, Global Head of Cell Therapy, HemaCare.
David Smith, Head of Innovation and Engineering, Hitachi Chemical Advanced Therapeutics Solutions.
This work is licensed under a Creative Commons Attribution- NonCommercial – NoDerivatives 4.0 International License.
About HemaCare:
HemaCare is the global leader and trusted brand in the customization of human-derived biological products and services for biomedical research, drug discovery, and cell therapy process development. The company’s network of FDA-registered, GMP/GTP-compliant collection centers ensure fresh donor material is available to customers and for use within HemaCare’s research and GMP-compliant isolation laboratory. Human biological material including peripheral blood, bone marrow, and cord blood is isolated into various primary cells types for fresh and frozen distribution. For over 40 years, HemaCare has developed an extensive registry of repeat donors and provides human-derived primary blood cells and tissues for biomedical and drug discovery research and cell therapy clinical trials, and supports commercialization with apheresis collections, directly enabling customers to advance both autologous and allogeneic cellular therapies.
Our recently opened new, state of the art facility enables HemaCare to provide GMP collections and GMP processing. In addition to a newly renovated, larger footprint and donor collection center, the facility has four cleanroom suites, which are both FDA and EMA compliant. Combined, the new facility provides an optimized process flow which reduces risk (handling and time) between steps to ensure a flexible and scalable production environment to support industry demand.
About Hitachi Chemical Advanced Therapeutics Solutions (HCATS):
 Hitachi Chemical Advanced Therapeutics Solutions, LLC (HCATS), is a wholly owned subsidiary of Hitachi Chemical Company, Ltd. (Hitachi Chemical) representing Hi¬tachi Chemical’s Regenerative Medicine Business Sector in the United States. HCATS leverages two decades of experience exclusively focused on the cell therapy industry. It provides contract development and manufacturing organization (CDMO) services at current Good Manufacturing Practices (cGMP) standards, including clinical manufacturing, commercial manufacturing, and manufacturing development. For more information about these services, please visit www.pctcelltherapy.com
Hitachi Chemical Advanced Therapeutics Solutions, LLC (HCATS), is a wholly owned subsidiary of Hitachi Chemical Company, Ltd. (Hitachi Chemical) representing Hi¬tachi Chemical’s Regenerative Medicine Business Sector in the United States. HCATS leverages two decades of experience exclusively focused on the cell therapy industry. It provides contract development and manufacturing organization (CDMO) services at current Good Manufacturing Practices (cGMP) standards, including clinical manufacturing, commercial manufacturing, and manufacturing development. For more information about these services, please visit www.pctcelltherapy.com
HCATS is investing heavily in a number of areas to accelerate the cell and gene therapy industry’s ability to mitigate some of the risk associated with the high variability of cell therapy products. Setting up a global footprint is vital for harmonization. With the recent acquisition of apceth in Europe, HCATS now has access to manufacturing capacity in the US, Asia and the EU through sites in New Jersey and California in the US, Yokohama in Japan, and Munich in Germany. Hitachi Chemical sites across the globe are looking closely at standardisation initiatives and are working with the standards and regulatory bodies in different regions to try to harmonise this industry around documented best practice for manufacturing.
Electronic systems are vital for the management of data flow throughout manufacturing and beyond. Starting with the patient, talking to the collection site, to the logistics company, to the cell therapy developer – this entire flow of data needs to be seamless. HCATS invests in electronic systems to help alleviate communication breakdowns and ensure chain of command and chain of custody.
Developing next-generation platforms to help control variation is going to be vital. It is another area of heavy investment by HCATS. There are many phenomenal people in this industry working to deliver the required solutions, but who require further investment and frequently need to hear more of the voice of the customer to ensure they are developing solutions that meet a need rather than developing ‘innovative’ solutions that unfortunately do not currently help the sector. This means that partnering with the right people – the experts – to combine experience and develop these new solutions together is going to be vital. Such partnerships are a priority at HCATS, as is ensuring we can provide our clients and our partners with the best tools available. With the recent announcement of Hitachi Chemical’s intention to acquire Apceth in Europe, HCATS will soon now have access to manufacturing capacity in the US, Asia and the EU through sites in New Jersey and California in the US, Yokohama in Japan, and Munich in Germany.