Improving upon legacy vector and plasmid bioprocess technology for tomorrow’s advanced therapies
Cell & Gene Therapy Insights 2021; 7(3), 811–829
10.18609/cgti.2021.113
It is undeniable that plasmids are of great importance to the biotechnology industry. Plasmid technology not only supports existing modalities but is key for the development of next-generation cell and gene therapies, along with the emerging field of mRNA therapies. Modern purification technologies for plasmid DNA and viral vectors are poised to improve both productivity and speed compared to legacy methods. This article will focus on the importance of plasmids, along with modern processing methods for plasmid DNA and vectors, including solutions from Cytiva that can offer flexible, robust results with significantly improved productivity as compared to conventional methods.
Plasmids: the basics
Plasmids are a natural part of many cells, and are comprised of small extrachromosomal plasmid DNA (pDNA) molecules. They are carriers of genetic information, enable functions including communication between cells, and provide properties such as resistance to antibiotics. Within the field of biotechnology, plasmids are predominantly of interest as vectors that can be used as tools to clone, amplify, and express genes. GMP-grade plasmids can also be used for in vivo applications such as DNA vaccines and gene therapy.
Plasmids come in many different shapes, forms, and sizes. Supercoiled plasmids in the size range of 5–20 kbp are of key interest to the biotechnology industry, and are most commonly used in bioprocess applications. Driven by new modalities, growth and market interest in plasmids has increased significantly in the last few years. As of 2020, the global plasmid market represents more than $80 million per year, and is growing at a rate of over 15% per year.
Plasmids are of key importance to the cell and gene therapy industry, and represent the starting point for many different modalities. They are used as a platform for many approaches found in laboratories around the globe. The entire biopharmaceutical industry has been built on the invention of recombinant DNA technology, which requires plasmids for the development of new expression systems. In both cell and gene therapy, plasmids are used to transfect cell lines to produce different viral vectors of interest. In order to develop mRNA-based therapies and vaccines, plasmids are used as templates for the enzymatic production of mRNA. For so-called DNA vaccines, GMP-grade plasmids is the active pharmaceutical ingredient (API).
Although plasmids are a common denominator within the cell and gene therapy industry, plasmids themselves are not one-size-fits-all, and one plasmid process cannot fit all needs within the industry. Requires volumes and quality of plasmids vary greatly depending on application area (Figure 1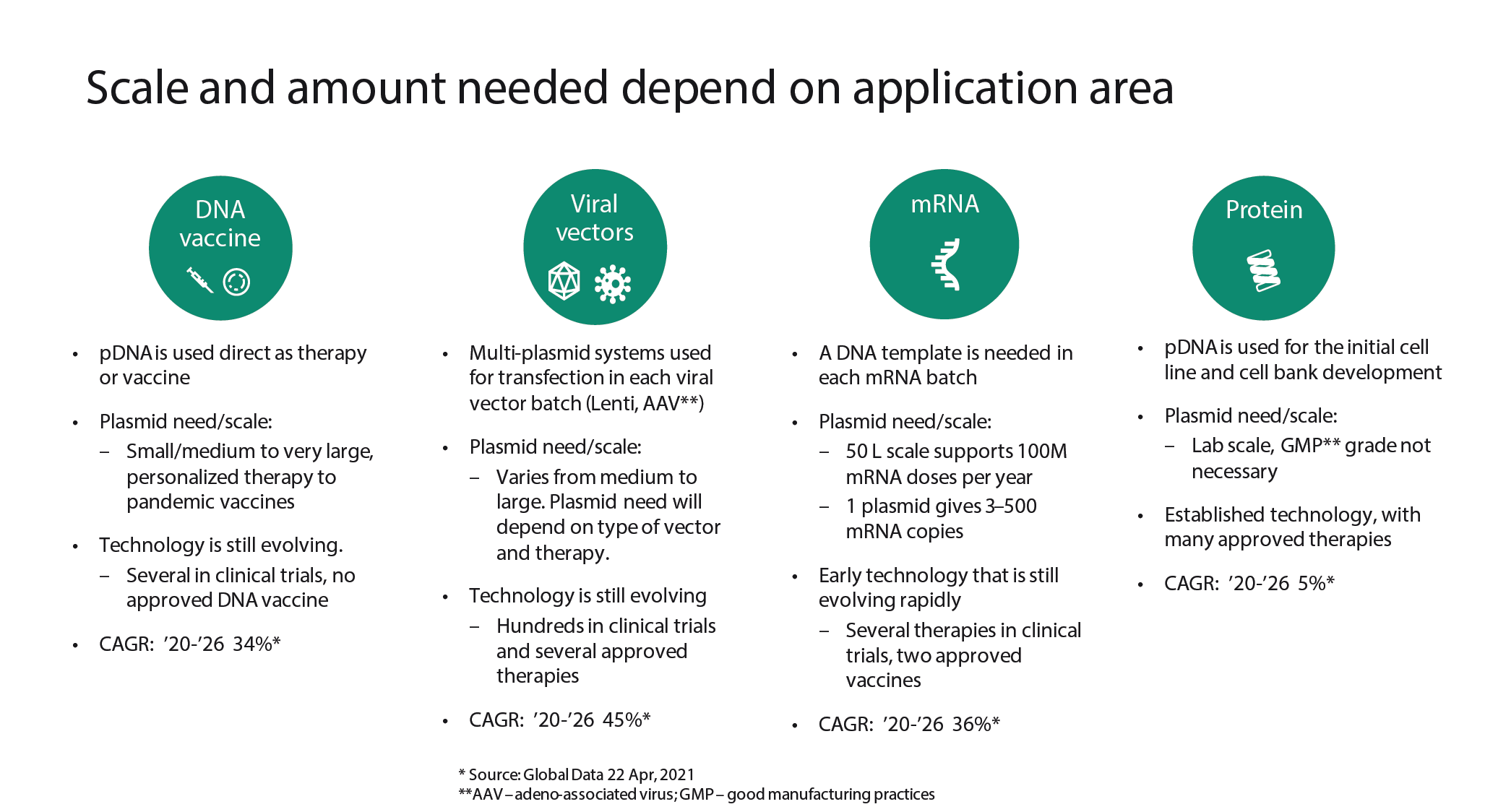
It is important to note that DNA vaccines are still in the early stages of development. If they achieve high success on the market, this may potentially drive the need for significant volumes of plasmids, as for this type of application the plasmid is the API, and purity requirements are therefore extremely high.
Quantity is not the only consideration – today, the field of gene and cell therapy requires not only large quantities of plasmids but also several different plasmids for each cell transfection. It is not uncommon for each viral vector to require up to three or four different plasmids – not including the plasmid containing the gene of interest – to develop a functional viral vector.
The emerging fields of mRNA-based vaccines and therapies require a plasmid as a template for the enzymatic in vitro transcription reaction. Each plasmid template may give rise to several hundred copies of mRNA, meaning smaller volumes of plasmid could generate larger quantities of mRNA molecules. When considering the plasmids needed for the design and development of new expression systems for different recombinant proteins, only very small amounts of plasmid are required.
There are also newer technologies that may change the future plasmid landscape. The current cell lines designed for viral vectors may be modified in such a way that only the plasmid containing the gene of interest is required, which could drive down volume demands. Rolling circle amplification (RCA) is an alternative process to the more traditional E. coli-based expression systems, which would allow for truly cell-free expression, and this option may be more suitable for small-scale GMP and personalized applications, while also potentially allowing for shorter timelines. Other options could include synthetic DNA templates or re-use of templates. Self-amplifying mRNA techniques are also reasonably advanced, and could, if they are shown to be successful in clinical trials, mean less need for plasmid as template for production of mRNA.
Large-scale plasmid biomanufacturing options
A biomanufacturing enterprise includes the process, facility, resources available, and infrastructure. These elements are integrated, and influence each other in significant ways. When designing a plasmid process, the primary objective is typically to manufacture a certain mass of supercoiled plasmid to the right specifications for the intended application. Challenges include meeting the different requirements needed for different plasmids and applications, meeting yield and purity goals, and ensuring access to manufacturing capacity. Designing a process to meet scale and purity goals, leveraging modular and single-use strategies to provide flexibility, and using integration solutions to ensure compliance and efficiency, are all strategies that can be employed in plasmid GMP manufacturing to ensure success. Cytiva’s FlexFactory™ platforms and KUBio™ facilities are offerings that can be built around a process and its specific mass balance, and process design services are available to better support process understanding.
From a downstream process perspective, there is typically a complex and challenging feed from the E. coli reactor, that may contain up to 3% of the desired plasmid along with impurities that need to be removed. Cytiva has a downstream process in place which is based on three key chromatography steps: achieving RNA reduction by size-exclusion chromatography, a thiophilic step to separate the active supercoiled form from the non-active open circular form of the plasmid, and finally, an ion exchange step to remove endotoxins.
Figure 2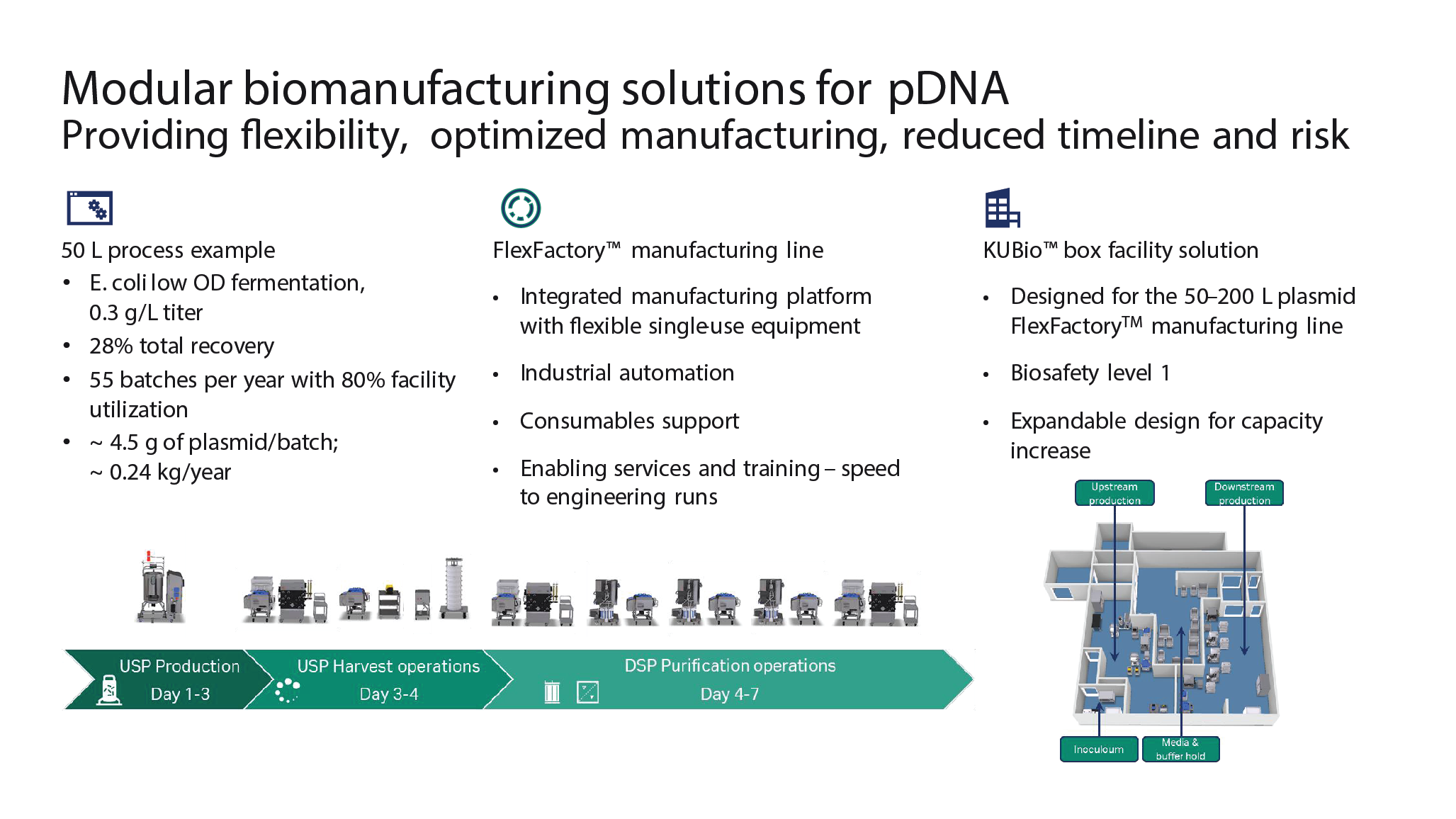
Process intensification with fiber-based plasmid purification
Looking to the future of plasmid production, there are incoming technologies and formats which may support higher productivity processing of both plasmids and other large target molecules.
One of the key challenges with purification of large target molecules is that they may not be able to enter the pore structure of a porous bead, resulting in low overall capacities and productivities. The new Fibro technology recently launched by Cytiva may offer a solution for such large target molecules and processes (see Box 1).
| Box 1 |
Fibro chromatography in downstream processing of AAV.Due to the importance of AAV to the field of gene therapy, Cytiva has prioritized the development of a new AAV capture product. Higher capacity, process intensification, and improved productivity in AAV manufacture are all prerequisites to allow for the treatment of large patient populations, and make promising and curative therapies more accessible in a sustainable and effective manner.Low titers are a key challenge in downstream processing of AAV, and may result in the need to load very large sample volumes onto a capture column. As a result, it may be necessary to over-size the affinity resin column to allow processing in a reasonable timeframe. An alternative is to concentrate the feed with a tangential flow filtration (TFF) step, but this takes time and will impact overall recovery. Resins have a great capacity for proteins and other smaller compounds that can access all of the available ligands within the pores of a resin chromatography bead. When purifying larger entities, only a limited percentage of the target entities can access the ligands that are on the interior of the chromatography bead, and therefore capacity drops with entity size. Some biological entities such as lentiviruses, AAV, exosomes, plasmids, mRNA, and liquid nanoparticles (LNPs) are much larger and can only bind on the outer surface of the bead, resulting in relatively low resin capacity. In contrast, Fibro chromatography uses electrospun cellulose fibers, which generate a structure that is more porous than a chromatography resin, allowing AAVs and other large entities to bind throughout the material. As seen in Figure 11 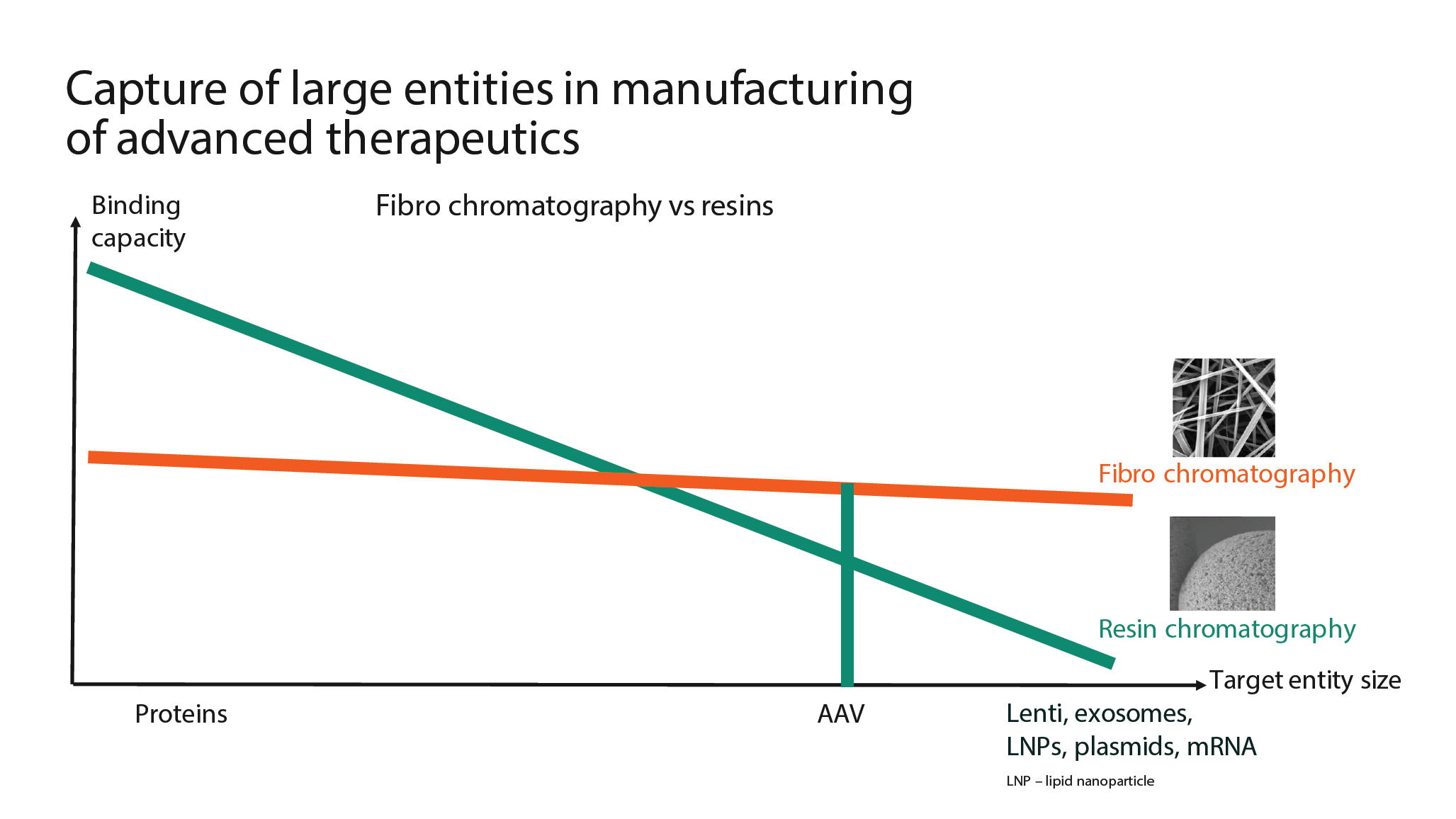 During the capture step of downstream processing, binding capacity varies with target entity size., this provides relatively stable capacity regardless of target entity size. During the capture step of downstream processing, binding capacity varies with target entity size., this provides relatively stable capacity regardless of target entity size. |
Case study: conventional bead format versus novel Fibro format
Significant benefits can be achieved by coupling the same ligand to a different porous structure, allowing for fuller access to the surface area. As shown in Figure 3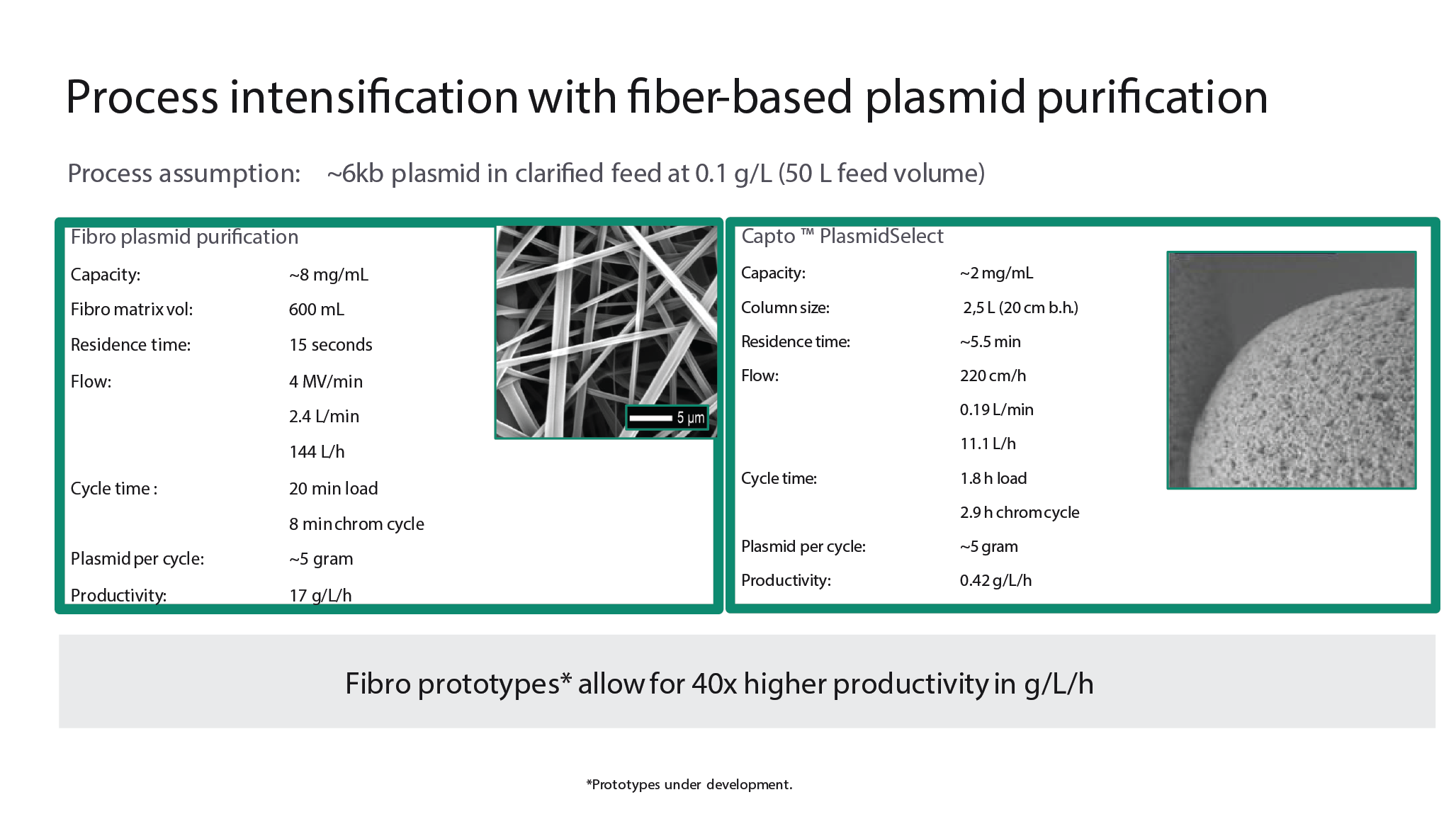
A 2.5-liter ready-to-process column packed with Capto™ PlasmidSelect and a 600 ml Fibro cassette modified with the same Capto PlasmidSelect ligand were compared, using a feed of 50 liters of 6 kbp plasmid, at 0.1 g/L. Fibro plasmid purification offered up to 40 times higher productivities due to the rapid cycling combined with the high accessibility of the surface structure. This novel format could be suitable not only for plasmids, but for large target molecules in general, and may offer significant improvements in both productivity and process economy.
Bioprocessing of adeno-associated virus (AAV) vectors
Viral vectors can be used for a variety of purposes, including vaccines, oncolytic therapies, or gene therapies. Viral vectors can also be used in cell therapies as reagents and, regardless of the final application, there are some common themes in the production process.
Everything starts with expansion of the producer cells – in this case, HEK293 cells. Once the cells have been expanded the virus must be introduced, which can be done via the infection or transfection route. The virus then propagates in the cells, before being released and purified. Finally, there are a series of formulation, fill and finish steps. Cytiva’s AAV production process is shown in Figure 4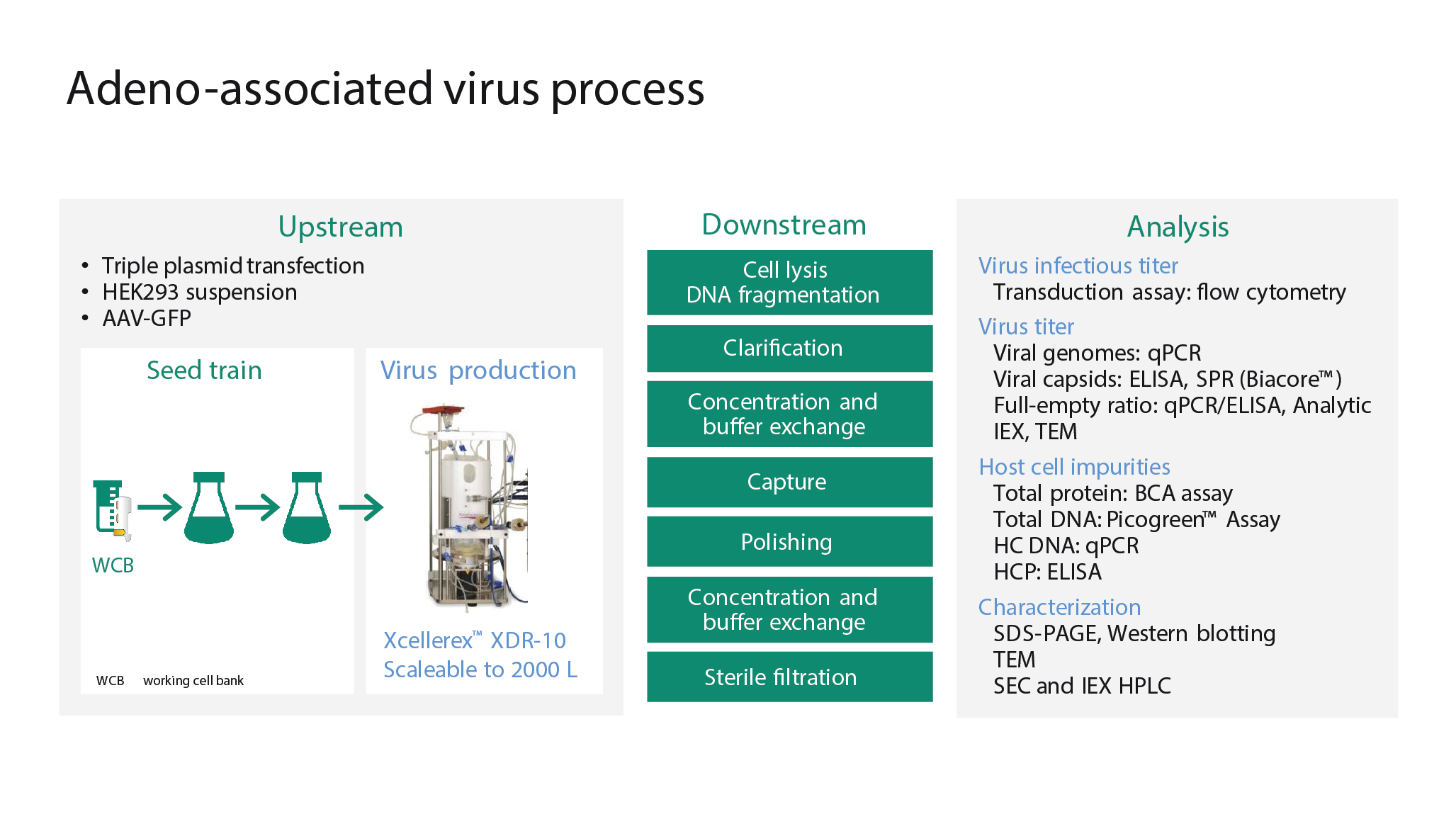
Briefly, a triple plasmid transfection system is used to transfect HEK293 cells with the green fluorescent protein (GFP) gene. A vial from the working cell bank is thawed and the cells are expanded in shake flasks to the volume required to inoculate the production bioreactor (in this case the Xcellerex™ XDR-10 single-use stir-tank bioreactor). Further downstream, scalable and robust technologies based on filtration and chromatography technologies are used for purification. A range of orthogonal analytics is used to ensure performance.
Upstream processing
Cytiva’s upstream strategy was based on the need to adapt the host cells to serum-free suspension cell culture. A number of different animal-origin-free cell culture media were validated, since avoiding any animal-derived components offers a clear regulatory advantage.
To optimize the triple plasmid transfection procedure, design of experiments was used to assess parameters including:
- Cell density and volume at transfection
- Plasmid concentration and ratio
- Transfection reagent (PEI)–plasmid ratio
- Incubation time of mix prior to transfection
- Temperature
- Supplement
- Time to harvest post-transfection
Success criteria included transfection efficiency over 70%, and AAV titer of at least 106 TU/mL or 109 VP/mL. The resulting optimized transfection protocol can be seen in Box 2.
| Box 2 |
Optimized transfection protocol.
|
The optimized protocol produced around 1011 VP/mL both in the shake flasks and in WAVE™ 25 and XDR-10 bioreactors. Productivity was consistent for serotypes AAV2, AAV5, AAV8, and AAV9. The process is also effective at larger scales: Cytiva customer Homology Medicine scaled up this transfection process from 2 liters to 500 liters, with good linear scalability.
Downstream processing
A similar step-by-step optimization was followed for development of a downstream process. In particular, two chromatography steps are critical for the performance of the overall process. First, the virus was captured on a chromatography column with Capto AVB affinity resin, which binds to several different AAV serotypes, including AAV2 and AAV5. The material was loaded onto the column, then washed and eluted. Cytiva’s ÄKTA pure protein purification system was used along with the Capto AVB resin packed in HiTrap™ columns. With a concentration factor of ∼100-fold, recoveries of between 70% and 80% were achieved. Results achieved with this approach are shown in Figure 5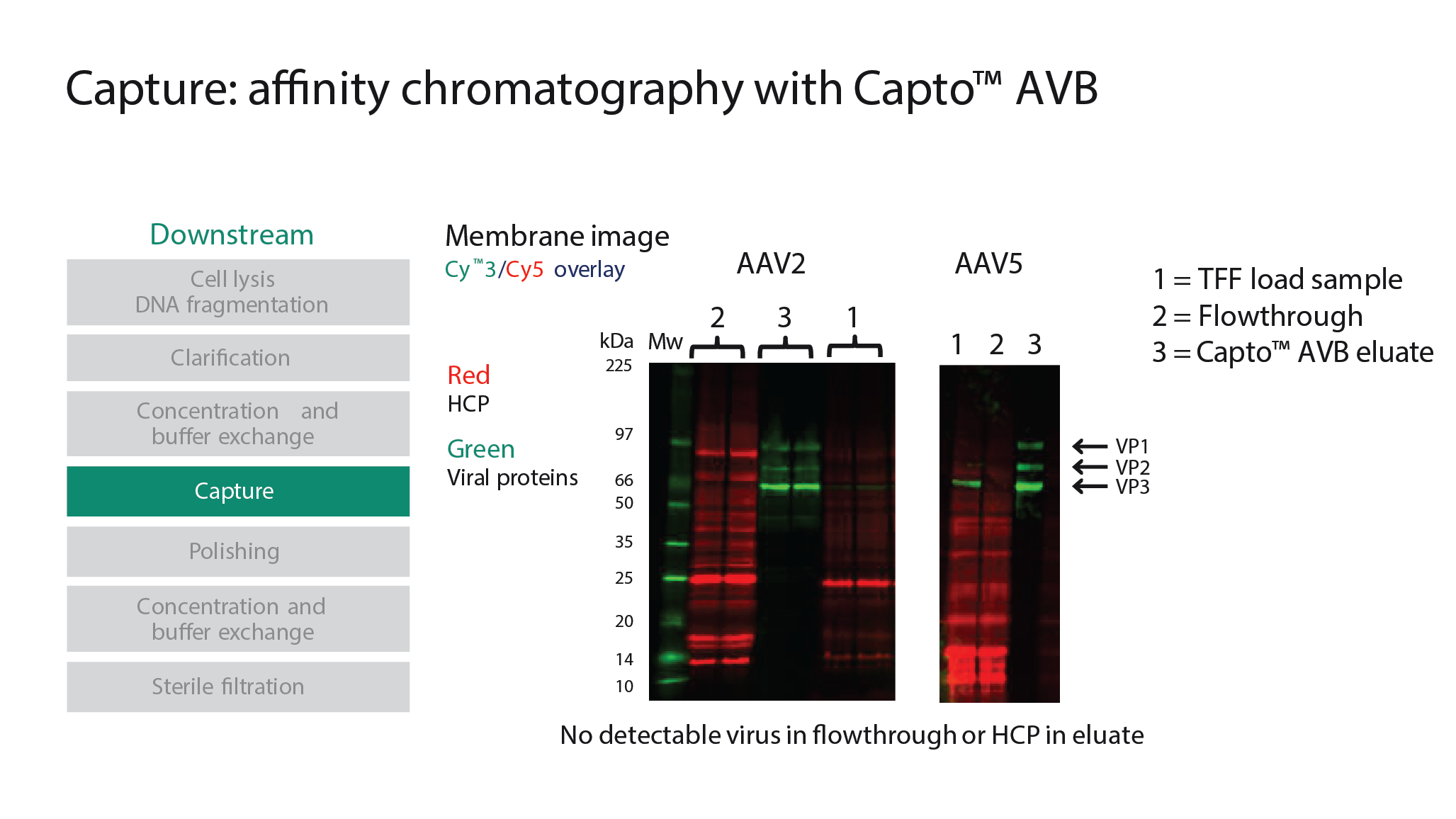
The Capto™ Q ImpRes, an ion exchange resin, was then used in the polishing step in order to reduce empty capsids. Polishing can prove to be a challenging step to optimize, and there are a number of critical parameters which must be evaluated and optimized. Along with ensuring the maximum amount of full capsids are present in the starting material, optimization of pH, magnesium chloride, additives, and wash gradients is required. When considering recovery of the viral genomes and percentage of full capsids, some trade-off regarding recovery may be necessary, depending on the target for enrichment of full AAV capsids.
Improving analytics
Analytics are critical to process development for viral vectors. Cytiva has developed a new AAV quantification assay using the Biacore™ T200 system (Figure 6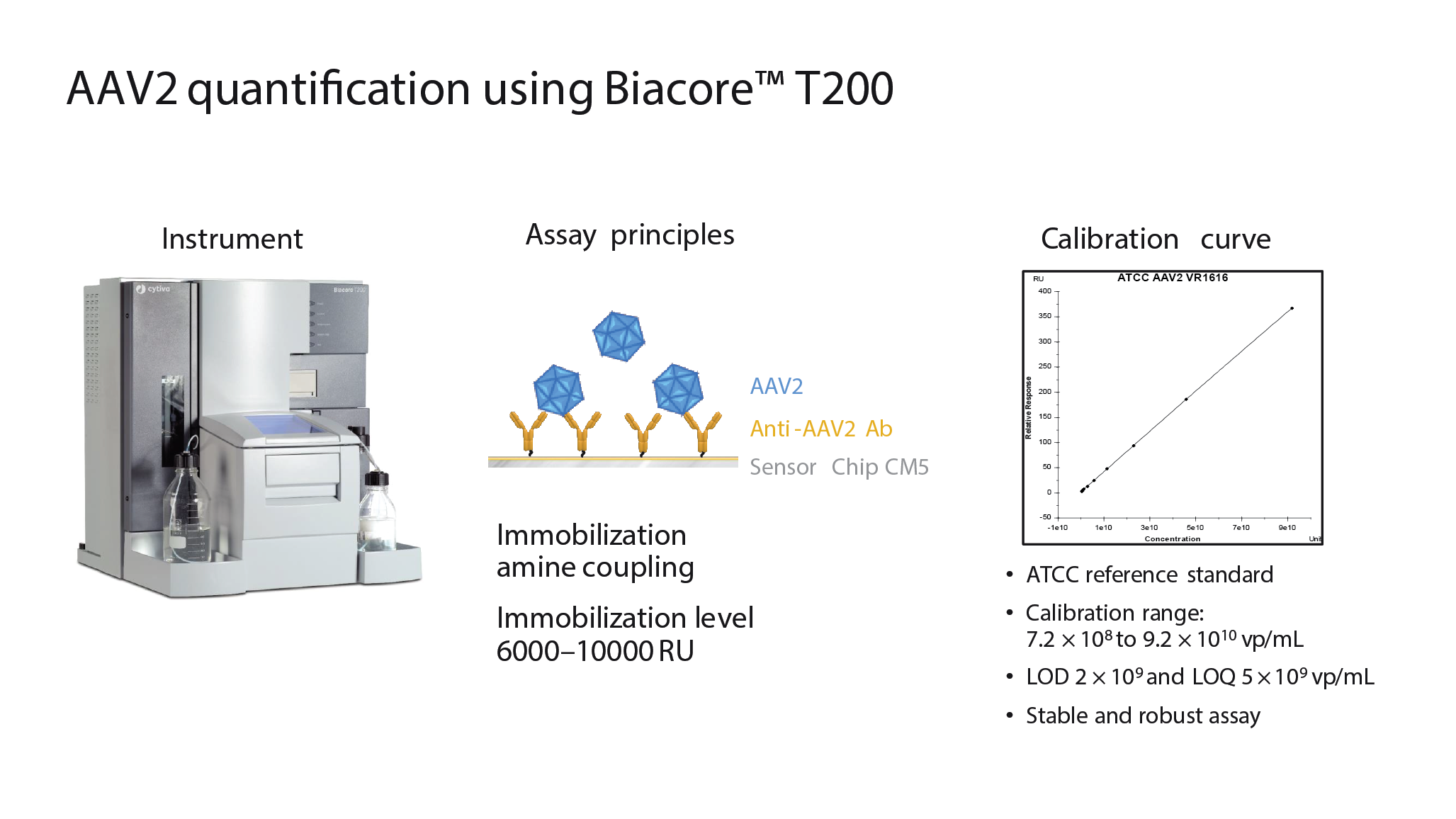
The assay is based on immobilizing anti-AAV antibodies to the sensor chip in the Biacore instrument via amine coupling. As the sample flows over the chip, interactions between the antibody and the virus can be detected. In the above example, anti-AAV2 antibodies were used, but other reagents can be utilized depending on the serotype being measured. To the right of Figure 6 is a calibration curve using an ATCC reference standard.
This is a stable and robust assay that has now overtaken the use of ELISA at Cytiva – Figure 7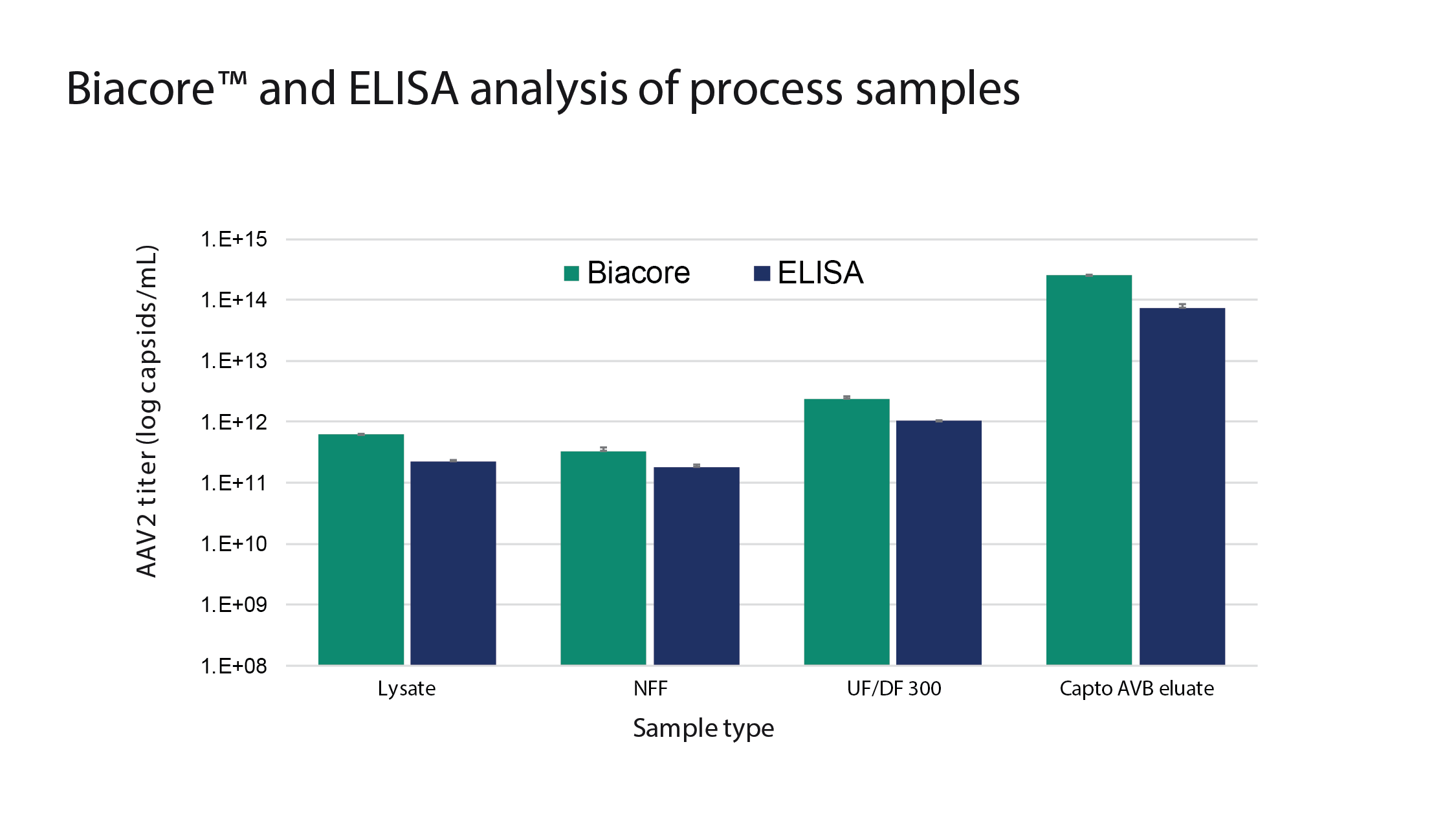
Conclusion
Plasmids are the cornerstone of modern biomanufacturing. As newer modalities enter the market, the need for a variety of different plasmids, in increasing quantities, will only grow. It is key for manufacturers to understand their plasmid requirements as early on as possible, to allow these needs to inform their process design and development. Offerings from Cytiva, including the FlexFactory, KUBio box, and Fibro chromatography, offer plasmid and viral vector bioprocess solutions that can be applied at different scales, to support the continued growth and development of both the latest advanced therapies and the biotechnology industry as a whole.
Developing Fibro
A chromatography cycle using Fibro is shown in Figure 8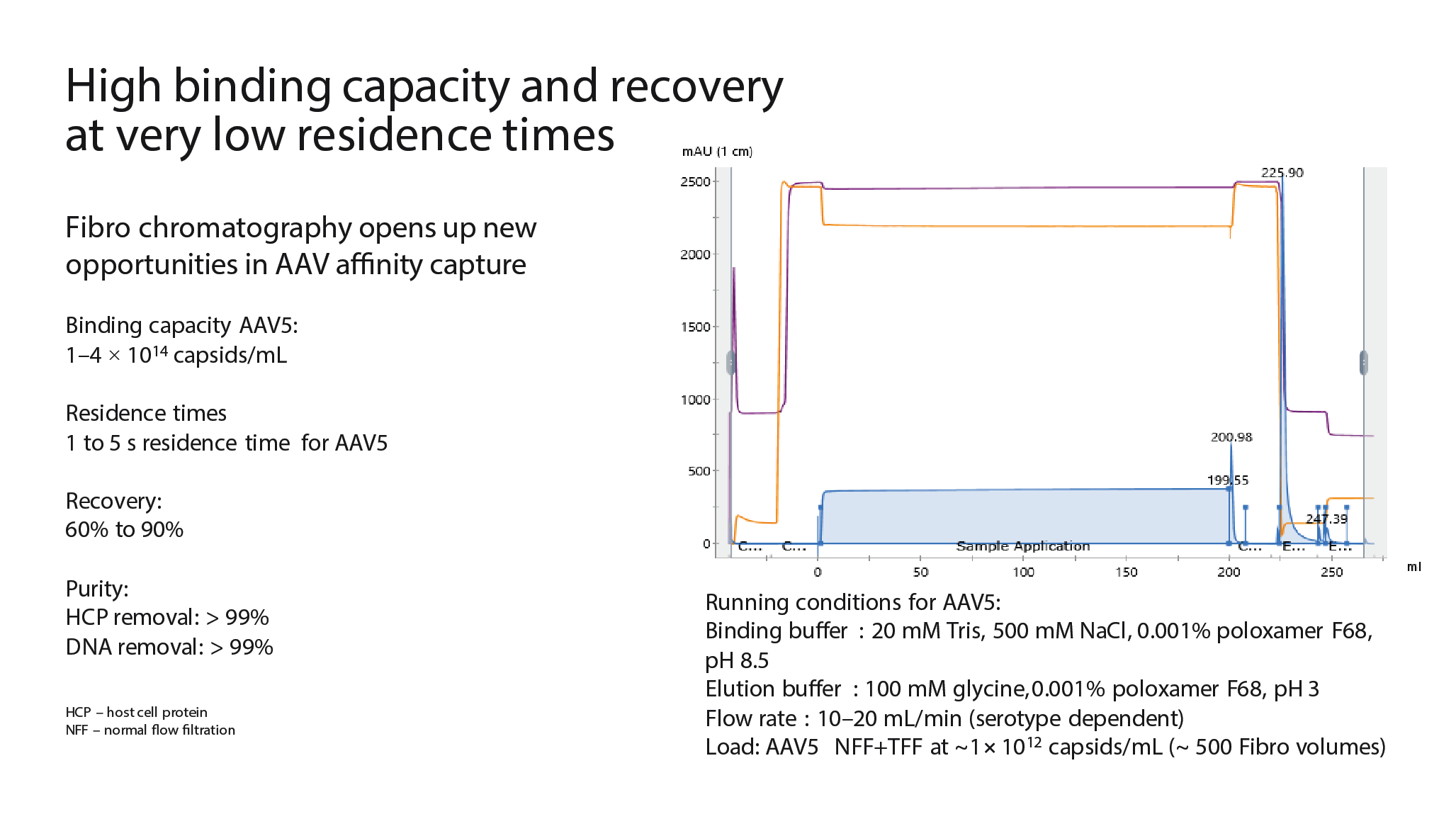
Clarification is important for achieving a high flow rate, and when developing Fibro for AAV, different clarification methodologies were considered. Normal flow filtration can be suitable for AAV feeds that do not use lysed cells. If lysed cells are used, which is often the case, depth filtration followed by normal flow filtration may be needed. There are also limitations when loading very large sample volumes with this clarification method, so for challenging feeds a charged depth filter or a precipitation step can be added to the midstream methodology.
Fibro prototype for AAV5 purification: Belief BioMed collaboration
Figure 9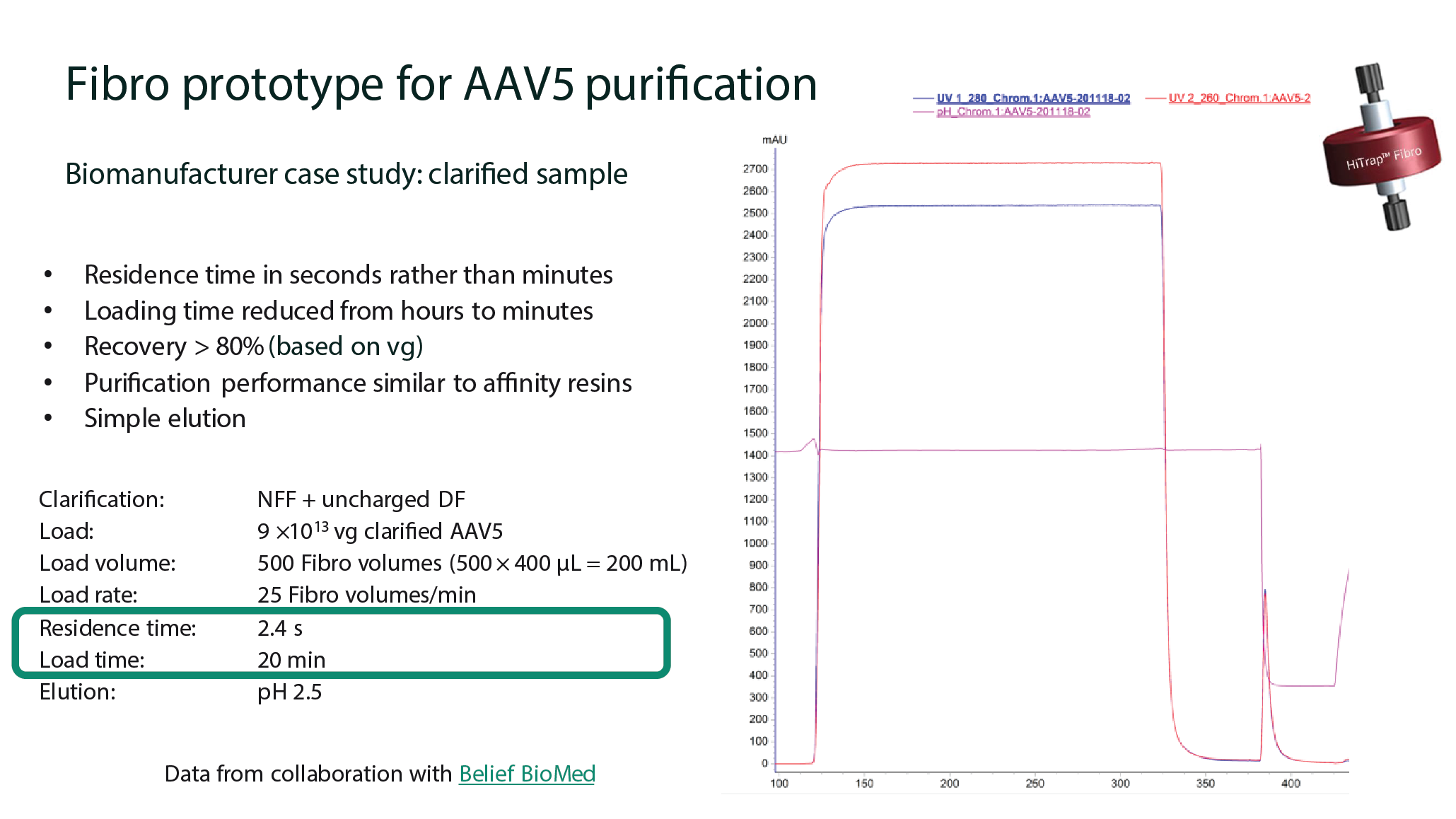
Figure 10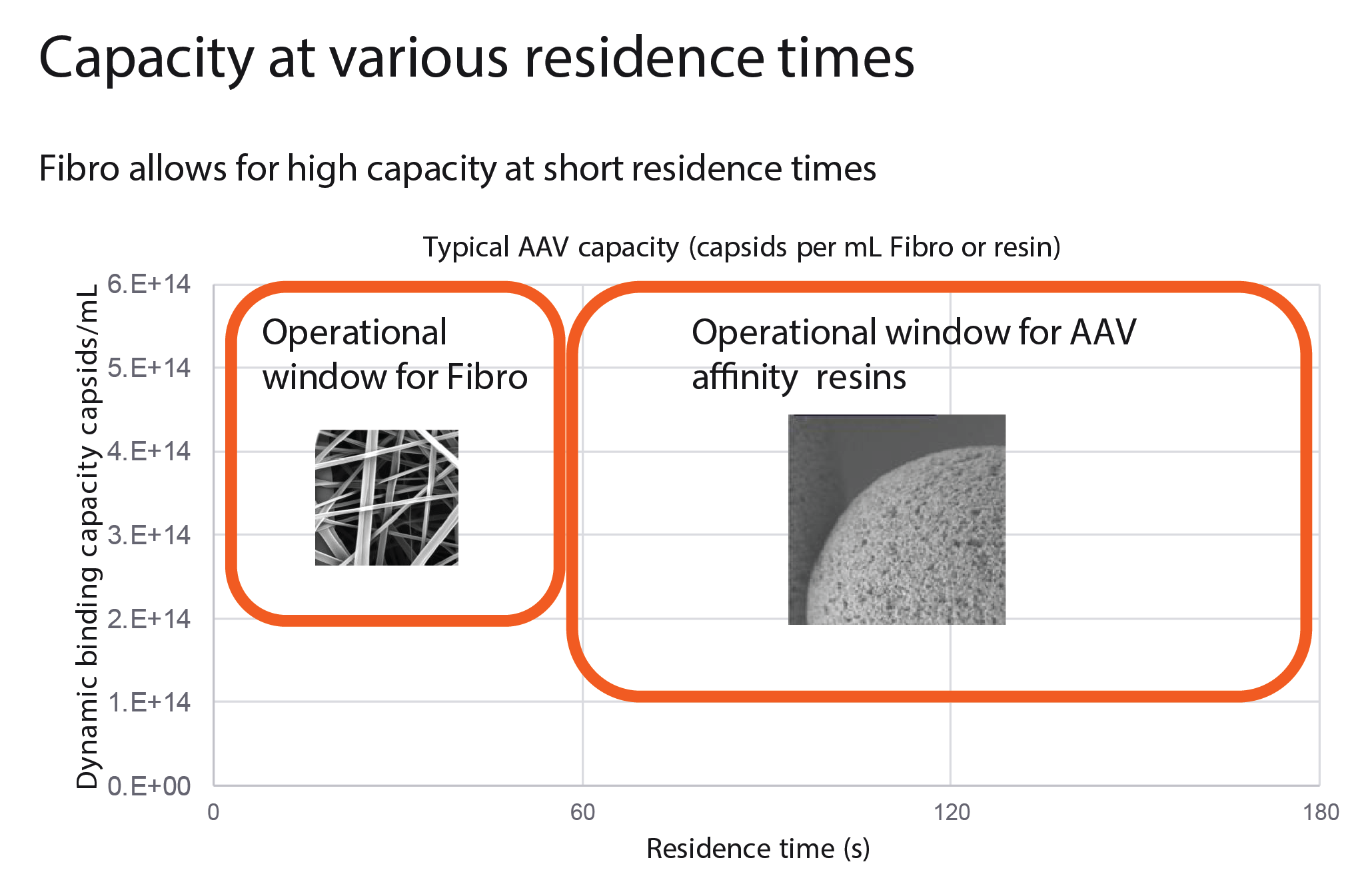
| Box 3 |
Case study: Resin collaboration with Cobra BiologicsThe need for a new resin in Cobra’s in-house downstream platform for plasmid purification was driven by an ever-increasing demand for a variety of plasmids. This put pressure on Cobra’s ability to process increasing quantities of plasmids in short timeframes. One of the key chromatography steps became the focus for improvement: the thiophilic or pseudo-affinity step, based on the PlasmidSelect Xtra Resin, which enables the separation between the open circular and supercoiled versions of plasmids.The key parameters that were targeted were improving the flow rate, lowering the back-pressure, enabling more flexibility in column packing with taller bed heights, and improving the dynamic binding capacity, which combined would allow for improved productivity and process economy. The legacy resin, PlasmidSelect Xtra, is designed on a base matrix developed in the early 1990s, with a relatively poor rigidity and an average bead size of ∼34 microns. The Cytiva custom resin team and Cobra decided to explore the option of developing a high-flow and high-capacity version of PlasmidSelect Xtra, based on a higher flow, agarose-based matrix. The two initial candidate base matrices were the Capto ImpRes and Capto ImpAct matrices, with average bead sizes of ~40 and 50 microns. The ImpRes base matrix has a smaller average pore structure as compared to the ImpAct base matrix. In total, 8 different prototypes were developed for testing and evaluation by Cobra, with ligand density as one of the key variables (Figure 12 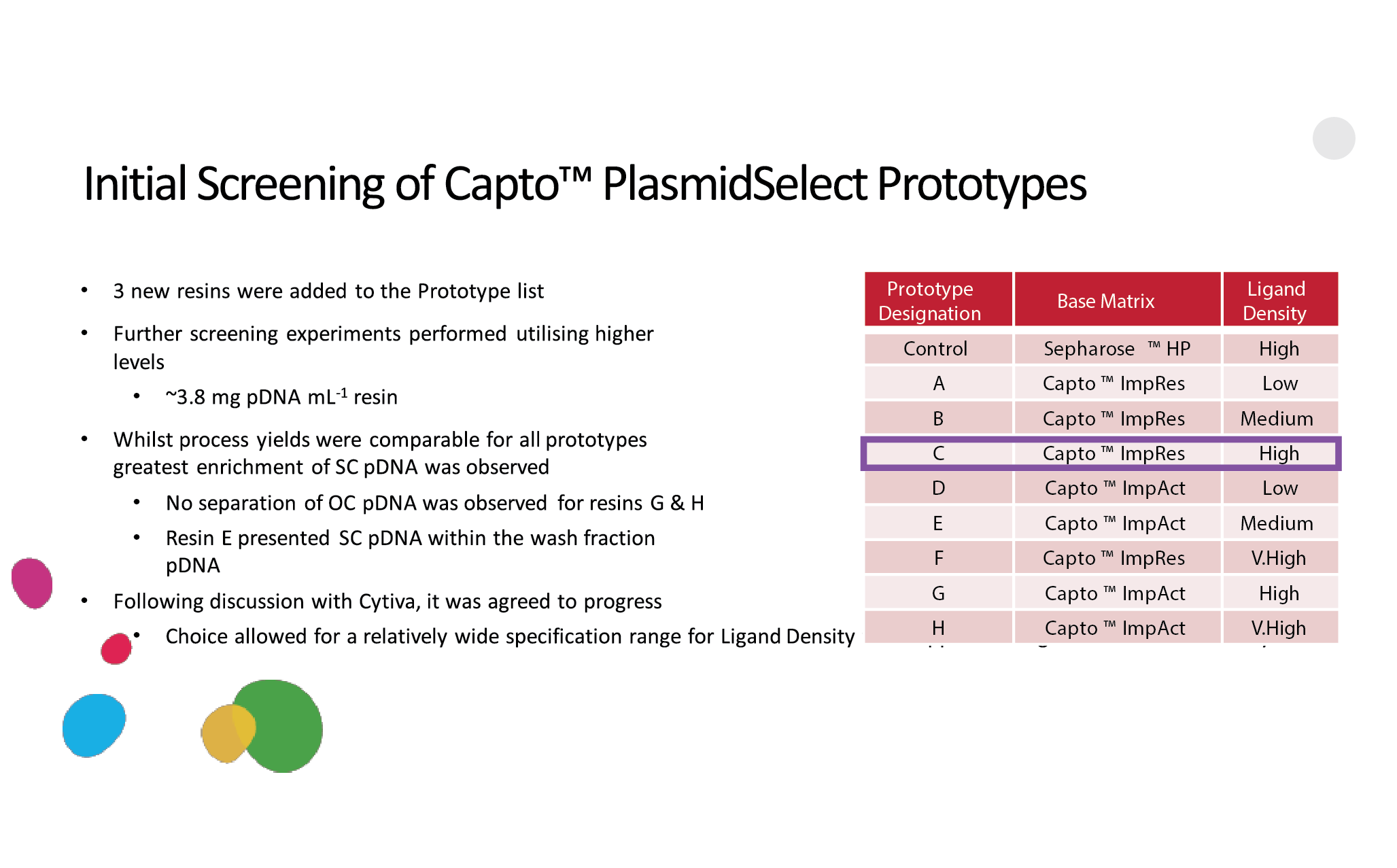 Initial screening of PlasmidSelect prototypes.). Initial screening of PlasmidSelect prototypes.).The prototype based on the Capto ImpRes base matrix combined with a high ligand density offered the best overall performance. In a benchmarking study, the results were in favor of the new Capto PlasmidSelect resin, as opposed to the legacy PlasmidSelect Xtra resin. The combination of a more rigid base matrix, combined with a higher ligand density, allowed for 44% less resin, almost 50% lower consumption of buffer, and roughly 14% faster processing (Figure 13 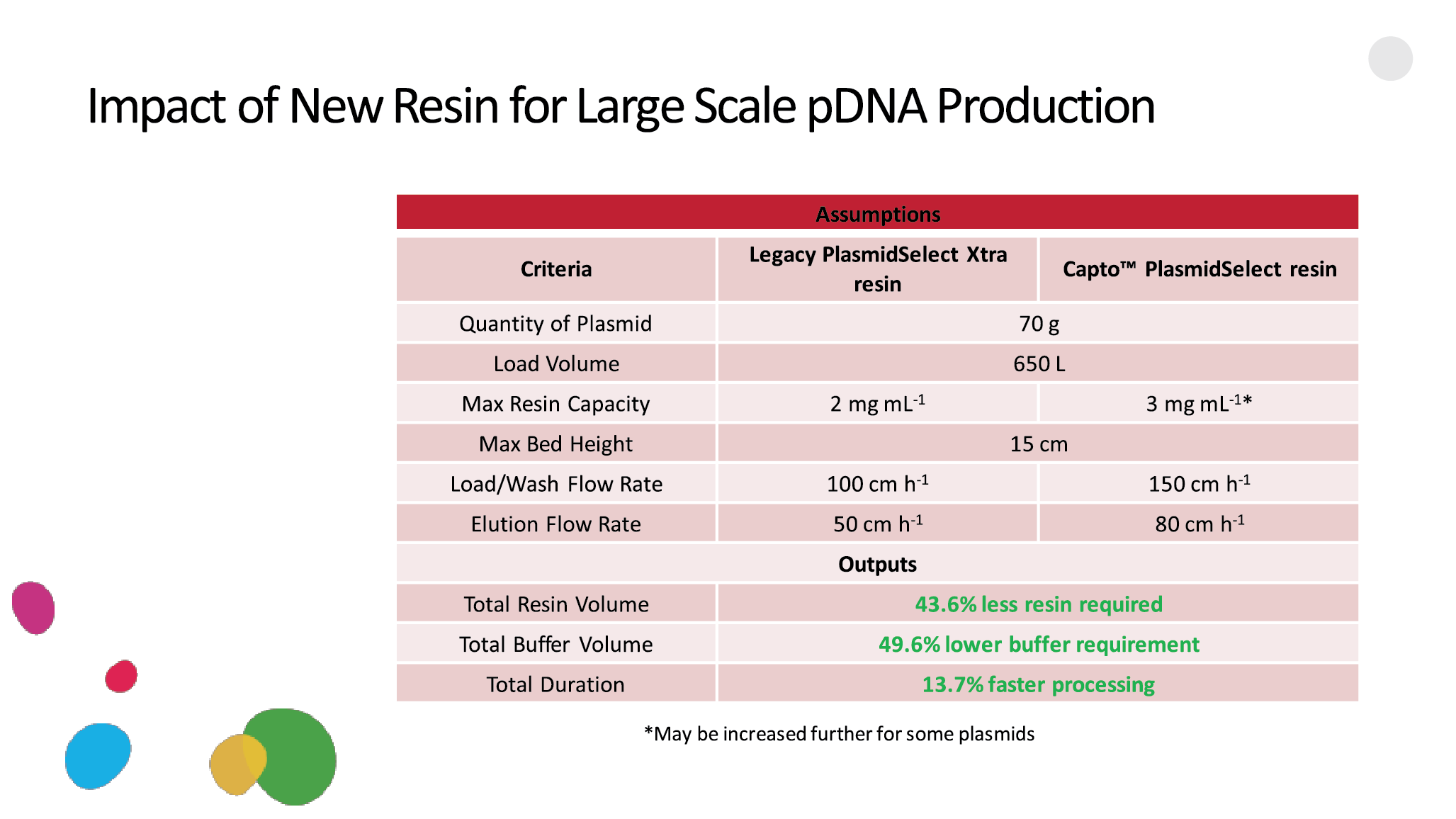 Results of a benchmarking study showing the impact of an improved resin for large-scale pDNA production.). This case study illustrates the impact a modern, high-capacity chromatography resin can have on production capacity. Results of a benchmarking study showing the impact of an improved resin for large-scale pDNA production.). This case study illustrates the impact a modern, high-capacity chromatography resin can have on production capacity. |
To summarize, the Fibro chromatography system enables significantly reduced process time for an affinity capture step. It provides an opportunity to not only speed up the loading and the capture step but also to potentially eliminate the need for TFF, which can positively impact both overall process time and yield. Fibro for AAV also provides similar recovery and purification performance as corresponding affinity resins. The Fibro system is simple to set up, and fits both laboratory equipment and GMP manufacturing instruments.
Q & A
Q Has the recent success of mRNA vaccines had an impact on pDNA processing?
HI: Due to the pandemic and the resulting success of mRNA vaccines, we can definitely see that everything around mRNA has sparked an interest. Since you need plasmids to make the mRNAs, we see significant interest in making a variety of different plasmids at different volumes and for different purposes.
PG: For mRNA vaccines it is a template, so the volumes of plasmid needed for each batch are significantly different, compared to transfection in the production of viral vectors, for example.
HI: Each plasmid can give rise to several hundred – maybe even up to 500 – copies of the mRNA. So even if you need larger quantities of mRNA, the volumes for the plasmids will still be rather small.
Q Will there be one plasmid DNA process in the future that fits all needs, or do you think the process will depend on the application?
HI: I don’t think so – as mentioned above, different plasmids could be used for different purposes.
To give an extreme example, if you want to have a plasmid that is going to be the active pharmaceutical component in a DNA vaccine, then it needs to be of GMP grade, whereas if you want to transfect cells, then perhaps the plasmid doesn’t have to be very pure. Then again, for mRNA I think it is important to have reproducible results from one lot to another, and in different volumes.
If you are going to make an expression system, you essentially need minute quantities of the plasmids that can be made on the lab bench. Whereas if you need three, four, or five different plasmids for cell and gene therapy applications, the quantities could be rather significant.
We need to keep in mind that we need to develop different processes, with a focus on different volumes and different qualities, depending on what they target or what they are designed for.
PG: One thing I will mention here is the size of the plasmid, and requests for much larger plasmids than were used before. This adds another dimension to the processing.
HI: This is a good point from Peter, and I think everyone has seen the recent progress of self-amplifying mRNAs. If they make it to the market, the corresponding plasmids are significantly larger than the ones we have seen up until now.
Q Can the transient transfection technology also be used for commercial manufacturing?
ML: Yes, it is already used in commercial manufacturing. Here once again, I would focus on the scale that is required. If you need huge scales, transient transfection may not be the optimal technology from a cost-of-goods perspective.
Q Are there other alternatives to the described polishing strategy if there are residual HCPs that need to be reduced?
ML: We have a product called Capto Core 400 which can be used for reduction of smaller host cell impurities, and also smaller DNA fragments and so on, that you can add in addition to the other steps if needed. What type of polishing strategy you choose also depends a lot on the serotype.
Q Is Fibro for AAV available to test?
PG: We had hoped that at least the smaller units would be launched by now, but the launch is delayed. We have several collaboration partners, and the aim is to make it available for others to test by the end of this year or early next year.
Q What is favorable with resins as compared to Fibro for AAV, in your opinion?
PG: The impurity reduction is similar as with Fibro, but I think overall you get better impurity reduction with a resin. The difference is quite minor, but it does make sense that it is usually a subsequent polishing step. If there is an advantage with resin, that may be the advantage. But it depends very much on your fill, your serotype, and the actual ligand employed.
Q Will the Fibro be applicable in GMP production environments?
PG: Yes – we aim to have these units available in sizes that are compatible with GMP manufacturing, and the sizes can process around 200 liters in one cycle.
The exact volume you can process in one cycle is of course very much dependent on the titer, but it is in that range, and the initial launch will include units of those sizes.
The Fibro technology will have larger titers launched, so there is an opportunity in the future to make even bigger units for AAV capture, possibly processing more than 1,000 liters in a cycle.
Q How does the yield performance compare for Capto PlasmidSelect Xtra versus legacy PlasmidSelect Xtra?
HI: Having a high throughput doesn’t mean anything unless you have good recovery and yield. I would say that the yield is on par with the legacy PlasmidSelect Xtra resin, keeping in mind here that we have almost identical bead size. The legacy is about 34 microns, the new about 40 microns, but the pore size is the same. It is the rigidity that makes up most of the difference, but yield I would say almost identical.
Q For purification of AAV, have you found any difference between the serotypes? For example AAV2 versus AAV5, and in the propensity to aggregate?
ML: In our hands at least, AAV2 has been more prone to aggregation than AAV5. We have seen a little bit of aggregation when it comes to AAV2, and you have to monitor the salt concentration in the buffers and so on to avoid that. We also sometimes use various additives, such as sugars or pluronic.
Q How critical is the initial UFDF step for AAV capture column performance, and why?
ML: When you are working with resin columns, in most cases you need to reduce the volume, otherwise the loading time will be very long. But of course, the UFDF step will also reduce impurities to a large extent, so you can get quite efficient purification in that single step. However, if you are working with affinity resins, you usually have very good selectivity with those, so you can load relatively crude material on them.
Q Does the Fibro remove intermediate capsids and provide more full capsids in your experience, and also is it known to reduce packaged extraneous host cell DNA?
PG: This depends on the ligand. For a standard AAV affinity ligand, there is no discrimination between full and empty capsids. But we have also made anion exchange versions and so on, and can get some resolution on this kind of Fibro structure in between full and empty.
As to whether it is known to reduce packaged host cell DNA – yes, certainly. If you have a ligand that doesn’t make the DNA co-elute, which most affinity ligands do, it is of course possible to remove this type of DNA.
Finally, do any of the presenters feel there will be a need for AAV production by continuous manufacturing process, inclusive of both upstream and downstream processes? If yes, would the Flex-ready platform provide a potential way to complete any continuous purification?
ML: This is a good question, and is debated quite a lot in the industry currently. The answer is yes, I think continuous manufacturing could have a place in these processes. Of course, the virus is relatively stable, so I don’t think you necessarily have to have continuous due to the stability of the virus. But you can reduce the size of the bioreactor, so there could be an advantage here.
PG: I think that continuous has potential. It may be other processes that are simpler to make in a continuous mode like lentiviruses that are being excreted, and also exosomes. Eventually, I think AAV may also move in that direction.
ML: Pete makes a good point – I think it is mainly the secreted serotypes that will be suitable for continuous manufacturing.
Some years ago, Cytiva’s custom resin organization was contacted by a leading CMO within the field of plasmids, Cobra Biologics, with a request to co-develop a resin allowing for higher productivities. Under the terms of the collaboration, the Cytiva team would develop different prototype resins for testing based on existing base matrixes and ligands at Cytiva, and Cobra would give valuable input on how these prototypes worked in their processes, and what the specification could or should be to satisfy both parties.
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2021 Cytivia. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: This article is a transcript of a previously published webinar, which can be found here.
Webinar published: May 18 2021; Publication date: Aug 3 2021.





