Evaluation of a microbioreactor system as a screening tool for optimizing lentiviral vector process development in suspension culture
Cell & Gene Therapy Insights 2021; 7(9), 1037–1046
10.18609/cgti.2021.136
With an increasing number of lentiviral vector (LVV)-based cell and gene therapy candidates reaching clinical trials, scalable suspension cell culture processes using stirred tank reactors (STRs) are needed to meet future demands. However, to cost-effectively scale LVV production in STRs requires process development which can be expensive and time consuming to perform in bench-top bioreactors. To address these issues, a multi-parametric approach for process development using a micro scale bioreactor system (Ambr® 15 cell culture system, Sartorius) was assessed. Since the medium exchange process step cannot be linearly or methodically scaled-down from a bench-scale STR to a microbioreactor due to system differences, this study focused on adjusting to those differences by developing and testing three different medium exchange protocols. The implementation of one approach (Process 2.0) using an automated cell settling medium exchange protocol produced results which closely aligned with an established LVV bench-scale process in transfection efficiency and productivity, as well as lowered variability between vessels in the cell culture workstation. In summary, this study demonstrates the suitability of the Ambr 15® system as a process screening tool which has the potential to reduce costs and timelines of the development of scalable LVV production systems in suspension culture.
LVVs for gene therapy
Lentiviral vector (LVV) systems are recombinant viral vectors used for delivering ex vivo and in vivo gene therapies into primary cells and are commonly used to correct a gene associated with a monogenic disease [1]Anguela XM, High KA. Entering the Modern Era of Gene Therapy. Annu. Rev. Med. 2019; 70, 273–288. https://doi.org/10.1146/annurev-med-012017-043332. In recent years there has been an acceleration in the number of clinical studies utilizing LVVs and by March 2021 according to ClinicalTrials.gov trial registry [2]Clinicaltrials.gOv Database Search for Clinical Trials Using Lentiviral Vectors.
https://clinicaltrials.gov/ct2/results?cond=lentiviral+vector+OR+lentivirus+OR+LVV&Search=Apply&recrs=b&recrs=a&recrs=f&recrs=d&age_v=&gndr=&type=&rslt= there were 646 active studies listed. This increase is driven in part by the successful use of LVVs in chimeric antigen receptor (CAR) T-cells as cell therapy to treat blood cancers and includes the FDA approved Kymriah [3]FDA press release: FDA approval brings first gene therapy to the United States
https://www.fda.gov/news-events/press-announcements/fda-approval-brings-first-gene-therapy-united-states to treat acute lymphoblastic leukemia (ALL). However, with Kymriah for example retailing at a list price of $475,000 US Dollars [4]Kymriah cost dispute goes to heart of pharma’s pricing row. Thepharmaletter 2018.
https://www.thepharmaletter.com/article/novartis-overpricing-kymriah-by-nearly-200-says-journal-report, these types of therapies are currently often prohibitively expensive. One reason for the high price tag is that the manufacturing Cost of Goods (CoGs) is high, with LVV production representing a large proportion of the costs [5]Comisela R-M, Kara B, Frederick H. Fiesser FH, Farida SS. Lentiviral vector bioprocess economics for cell and gene therapy commercialization.Biochemical Engineering Journal. 2021; 167: 107868.
https://doi.org/10.1016/j.bej.2020.107868Comisela R-M, Kara B, Frederick H. Fiesser FH, Farida SS. Lentiviral vector bioprocess economics for cell and gene therapy commercialization.Biochemical Engineering Journal. 2021; 167: 107868.
https://doi.org/10.1016/j.bej.2020.107868Comisela R-M, Kara B, Frederick H. Fiesser FH, Farida SS. Lentiviral vector bioprocess economics for cell and gene therapy commercialization.Biochemical Engineering Journal. 2021; 167: 107868.
https://doi.org/10.1016/j.bej.2020.107868. Therefore, as LVV is becoming more widely used, scalable and efficient processes across the production workflow are critical if LVV is going to be successfully manufactured to deliver a consistent, pure, high-titer product that is safe, efficient, and affordable. Thus, there is now a drive towards reducing LVV manufacturing costs to help ensure the commercial viability of many life-saving gene and cell therapies.
Addresing scalability to reduce LVV production cost
Looking to produce LVVs for commercial use, adherent cell-based processes in tissue culture flasks can robustly produce sufficient supply for clinical trials but are limited in their ability to scale to the demand required. Traditionally, these production processes are scaled-out by vessel number, but not scaled-up in size/volume, limiting the batch size to the number of flasks which can be successfully manipulated. For example, during an adherent LVV production process only approximately 40 L can be produced in 40 vessels, whereas production in a single 200 L STR increases the number of LVV units 5-fold volumetrically while the number of vessels to manipulate decreases significantly. This consolidation of vessels and scale-up in volume thereby increases batch consistency while reducing the CoGs per unit of LVV, respectively.
Adherent systems are therefore limited when moving from clinical trials to the commercial environment as they cannot be scaled-up but only scaled-out, increasing manufacturing complexity and processing time. To improve the use of adherent technologies for commercial manufacture, automated systems which use stacking T-flasks or roller bottles have been used, as well as fixed bed bioreactors [7]Macchiarulo E, Bassett P, Dudley M. Challenges and progress in lentiviral vector bioprocessing. Cell Gene Therapy Insights. 2018; 4 (10): 915–925. https://doi.org/10.18609/cgti.2018.091. However, since adherent cell culture has several disadvantages, including having an additional processing step to detach cells from the surface they are being cultured on and CoGs of LVV production being around 90% more expensive than single-use stirred tank bioreactors (STRs) [5]Comisela R-M, Kara B, Frederick H. Fiesser FH, Farida SS. Lentiviral vector bioprocess economics for cell and gene therapy commercialization.Biochemical Engineering Journal. 2021; 167: 107868.
https://doi.org/10.1016/j.bej.2020.107868Comisela R-M, Kara B, Frederick H. Fiesser FH, Farida SS. Lentiviral vector bioprocess economics for cell and gene therapy commercialization.Biochemical Engineering Journal. 2021; 167: 107868.
https://doi.org/10.1016/j.bej.2020.107868Comisela R-M, Kara B, Frederick H. Fiesser FH, Farida SS. Lentiviral vector bioprocess economics for cell and gene therapy commercialization.Biochemical Engineering Journal. 2021; 167: 107868.
https://doi.org/10.1016/j.bej.2020.107868, this has led to the use of suspension cell lines in STRs becoming more common for commercial manufacturing of LVVs.
All steps from a LVV production process in adherent culture systems can be substituted/replaced by corresponding steps in a suspension process. Processes in STRs can be scaled-up from the ~0.25 L lab-scale to a
00-2000 L commercial-scale through process optimization and development, offering the most flexible and cost-effective platform [5]Comisela R-M, Kara B, Frederick H. Fiesser FH, Farida SS. Lentiviral vector bioprocess economics for cell and gene therapy commercialization.Biochemical Engineering Journal. 2021; 167: 107868.
https://doi.org/10.1016/j.bej.2020.107868Comisela R-M, Kara B, Frederick H. Fiesser FH, Farida SS. Lentiviral vector bioprocess economics for cell and gene therapy commercialization.Biochemical Engineering Journal. 2021; 167: 107868.
https://doi.org/10.1016/j.bej.2020.107868Comisela R-M, Kara B, Frederick H. Fiesser FH, Farida SS. Lentiviral vector bioprocess economics for cell and gene therapy commercialization.Biochemical Engineering Journal. 2021; 167: 107868.
https://doi.org/10.1016/j.bej.2020.107868 to produce hundreds of liters of cell culture. Additionally, STRs also provide greater control of the culture environment than flask-based culture. Thus, these types of volumetrically-scalable suspension-based cell culture processes have the potential to meet future demands for LVV based therapies in indications with large patient populations [6]Martínez-Molina E, Chocarro-Wrona C, Martínez-Moreno D, Marchal JA, Boulaiz H. Large-Scale Production of Lentiviral Vectors: Current Perspectives and Challenges. Pharmaceutics. 2020; 12(11):1051. https://doi.org/10.3390/pharmaceutics12111051.
Process development of LVV production
Process optimization for LVV production is performed in bench-scale (2–5 L) bioreactors for eventual scale-up to pilot and manufacturing scale STRs (50–1000 L). This rapidly becomes cost-prohibitive when executing design of experiments or screening studies with numerous replicates to find the optimum process conditions due to the high cost of reagents and consumables in transient transfection-based processes. The work is also operationally intensive and requires substantial lab infrastructure to run multiple systems. With the goal of lowering cost while increasing throughput during development, a microscale bioreactor system was evaluated which could reproducibly perform the LVV production process to screen for changes that significantly affect yield and consistency.
Microbioreactors for process development
Single-use (SU) microbioreactors for screening mammalian cell culture conditions have been widely adopted in major biopharma companies including AstraZeneca and Merck for process optimization and development since 2010 [8]Lewis G. et al. Novel Automated Micro-Scale Bioreactor Technology: A Qualitative and Quantitative Mimic for Early Process Development. BioProcess J. 2010; 9(1): 22–25. http://dx.doi.org/10.12665/J91.Wales, [9]Moses S, et al. Assessment of AmbrTM as a model for high-throughput cell culture process development strategy. Advances in Bioscience and Biotechnology, 2012; 3: 918–927. https://doi.org/10.4236/abb.2012.37113. The drawback with many micro scale bioreactors is that they do not mimic the sparged, stirring action of a STR, and have no control over DO (dissolved oxygen) and pH inside the vessel. Additionally, not all micro scale bioreactors have the capacity for perfusion culture. With these, pH, DO and perfusion capabilities in mind, the Ambr® 15 cell culture high throughput automated microbioreactor system (Sartorius), an established technology for mimicking benchtop STRs [10]Hsu WT, et al. Advanced microscale bioreactor system: a representative scale-down model for bench-top bioreactors. Cytotechnology 2012; 64(6): 667–78.
https://doi.org/1007/s10616-012-9446-1, was selected as the LVV screening platform. The microbioreactor system mimics the characteristics of classical STRs at the miniature scale (10–15 mL) with each microbioreactor having its own agitation impeller and gases supplied by sparging or overlay. The system uses cost-effective, SU microbioreactors that are controlled by an automated cell culture workstation. Twenty-four vessels (12 vessels across two cell culture stations) can be operated simultaneously with the benefit of independent gassing for DO/pH control and built-in liquid handling.
High throughput tools with parallel processing capability, such as the Ambr® 15 cell culture system, help to address a major manufacturing bottleneck. The system can be used as a screening tool for process development, clone selection and effective media optimization in less time with reduced reagent use and labor saving [11]Lange I et al. Reduced Timelines in Early Process Development. Bioprocess International 2014;10: 34–37.
https://bioprocessintl.com/wp-content/uploads/2014/11/12-10-LangeVV-Secured.pdf. Furthermore, the system has been shown to be an excellent tool for mimicking perfusion processes in small scale to increase the viable cell density of Chinese Hamster Ovary cells used for monoclonal antibody production. The studies [12]Rees-Manley A, Evaluation of a Small-Scale Perfusion Mimic for Intensified Processes. Genetic Engineering & Biotechnology News 2019; 39 (12): 52–54
https://doi.org/10.1089/gen.39.14, [13]Ransome I, Implementation of Single-Use Miniature Bioreactors to Support Intensified Cell Culture. BioProcess International. 2020; 18 (3): 42–45.
https://bioprocessintl.com/wp-content/uploads/2020/04/18-3-Sartorius-Ian-Ransome-et-al.pdf used two approaches, a centrifugation method and a cell settling method for medium exchange, and these were used as the basis for methodology in this LVV study.
Proof-of-concept study: adapting a microbioreactor for LVV production
To determine if the micro bioreactor system could be used as a predictive screening tool for LVV production with a suspension cell line, a series of medium exchange protocols were assessed. Exchanging the culture medium post-transfection is essential due to cytotoxicity issues caused by transfection reagents, which can adversely affect titer [14]Tuvesson O, Uhe C, Rozkov A, Lüllau E. Development of a generic transient transfection process at 100 L scale. Cytotechnology. 2008; 56 (2):123–36.
https://doi.org/10.1007/s10616-008-9135-2. Several factors are easily scalable with the Ambr® 15 cell culture system using existing protocols including medium loading and conditioning, inoculation, gassing control strategy, agitation, and sampling. However, two operations: medium exchange and transfection liquid handling are less well defined for suspension cells and must be further developed for a robust, reproducible LVV production process.
The aim of this study was to implement and optimize an automated microbioreactor LVV production process for screening. The study focused on optimizing the medium exchange process step, a unit operation which can have a substantial impact on process performance and LVV titer. Additionally, the medium exchange process step cannot be linearly or methodically scaled-down from a bench-scale STR due to system differences, mainly the lack of analogous mini/micro scale medium exchange technologies, liquid handling mechanisms, and ability to consistently perform across 24 vessels in one process run.
Therefore, to adjust to system differences, an established proprietary LVV production process developed in a benchtop bioreactor was assessed in the SU microbioreactors and was redesigned through three iterations until it achieved culture characteristics and productivities comparable to bench-scale. These included achieving similar culture growth rates and viability, transfection efficiencies and infectious titer.
Medium exchange protocols
A proprietary HEK293T cell line was inoculated at a proprietary low cell density and cultured for a set duration until the proprietary target cell density for transfection was reached. The vessels were controlled at the targets of 37˚C, pH 7.0 ±0.2, and 50% DO by the Ambr® 15 system and with an impeller tip speed of 0.4 m/s. The viable cell density (VCD) and percentage cell viability were measured using an automated cell counter- (Vi-CELL™ XR, Beckman Coulter) throughout the culture duration. When cells achieved a target VCD, they were transfected using a proprietary transfection reagent with a proprietary four-plasmid system encoding the core proteins and transgene for LVV assembly. A 5th reporter plasmid was included encoding the Green Fluorescent Protein (GFP) gene. After a set duration post transfection (proprietary), the medium was exchanged to ensure robust transfection and production of functional LVV. Vector supernatants were harvested 48 hours post transfection and clarified by centrifugation (500 RPM for 5 minutes) to remove cells. LVV titer data for each production run was determined by qPCR of DNA extracted from a target cell line transduced with the LVV containing supernatants, reported as Transducing Units per milliliter (TU/mL). Productivity was also determined by quantitation of HIV-1 p24 antigen concentration (ng/mL) using a proprietary ELISA assay [15]Boucher E. et al. Qualification of a p24 ELISA Assay for Quantitation of Total Lentiviral Vector Concentration. Molecular Therapy. 2016; 24, (1) S187
https://doi.org/10.1016/S1525-0016(16)33282-8. Transfection efficiency (percentage GFP expression) was also measured by flow cytometry analysis on the production culture for each run at defined timepoints. These data were compared to the mean historic LVV titer data from 22 process runs in a benchtop bioreactor (2 L single-use bioreactors) generated using the same proprietary cell culture parameters.
Three medium exchange methods were evaluated in this study (See Figure 1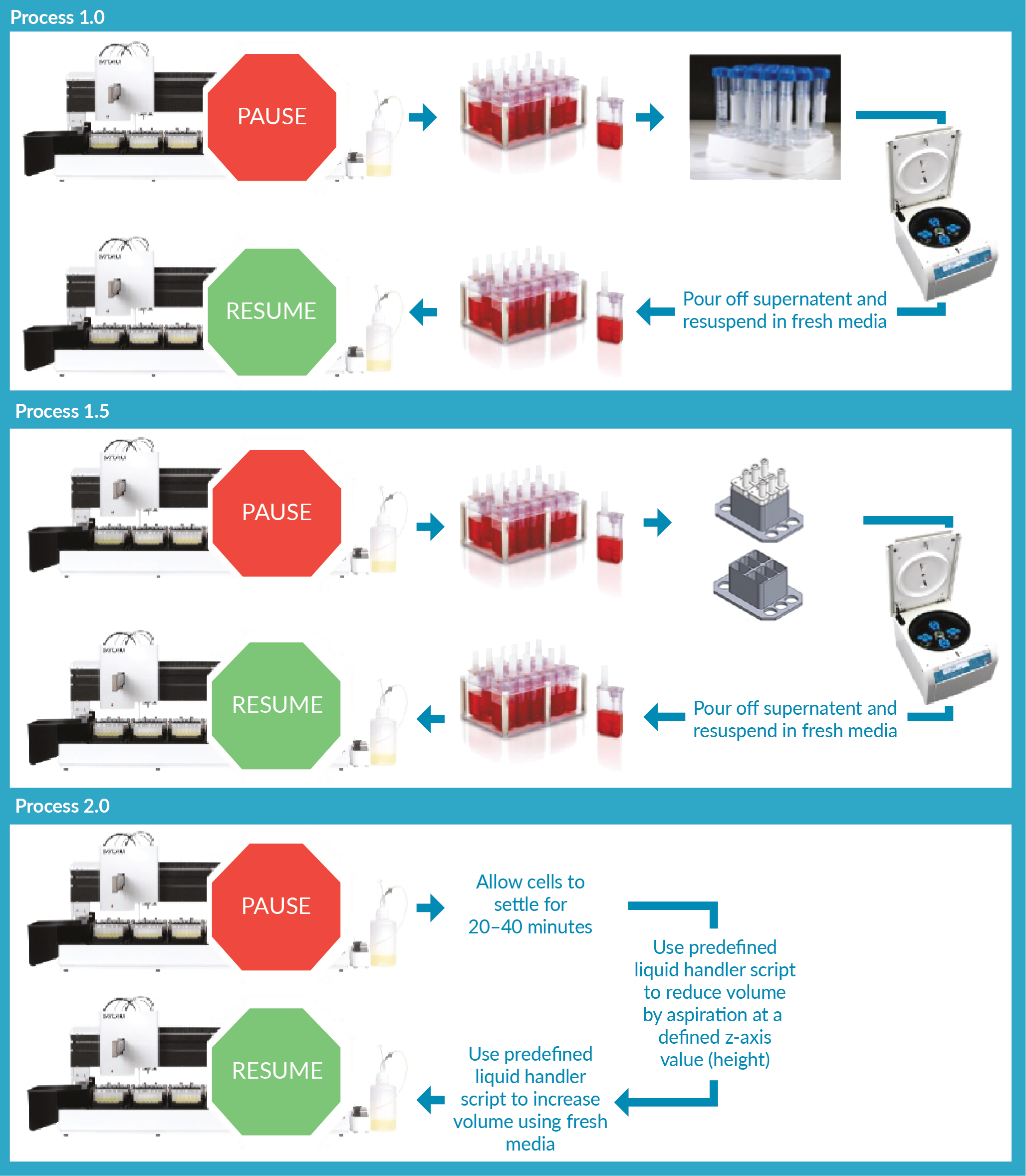
- Process 1.0: Microbioreactor vessels were manually removed from the cell culture station, the contents were transferred to sterile centrifuge tubes, these were centrifuged at 500 RPM for 5 minutes to spin down the cells into a pellet. The supernatant was then removed, fresh media added at an equivalent volume and cells were resuspended manually using a serological pipette. The culture volume was then transferred back to the microbioreactors by pipette, to be loaded back onto the automated bioreactor system.
- Process 1.5: The manual handling step of transferring the vessel contents into a centrifuge tube was omitted. Here, the microbioreactor vessels were centrifuged directly in specifically designed Ambr® 15 centrifuge adapters and were centrifuged at 500 RPM for 5 minutes to pellet the cells in one corner of the vessel. Then the supernatant was removed by pouring or aspiration, fresh media added, and cells were resuspended manually using a serological pipette. The microbioreactors were then loaded back onto the automated bioreactor system.
- Process 2.0: Since both Process 1.0 and Process 1.5 required manual handling which was time-consuming and posed a contamination risk, an automated cell settling process (Process 2.0) was developed. This involves pausing the agitation of the microbioreactors and allowing the cells to settle before exchanging a portion of the supernatant with fresh media. This is achieved by first drawing down the volume of each vessel in sequence using a 1-mL tip and then adding back an equivalent volume of fresh media once all the vessels of a cell culture station have been reduced in volume. A settling time of 20 minutes prior to spent medium removal of the first vessel was sufficient for the majority of the cells to settle to the bottom, limiting a decrease in cell density due to the removed volume. The Ambr® 15 system can only perform one drawn down at a time, which lead to the final vessel of a cell culture station being accessed after ~40 minutes of settling.
This process eliminated manual handling steps by using predefined liquid handling scripts in the Ambr® 15 software to facilitate the medium exchange by the liquid handler.
To determine if VCD or viability were compromised by the cell settling method for the medium exchange used in Process 2.0, VCD and viability data were measured before and directly after cell settling in three different experimental runs (designated experiment 1, experiment 2 and experiment 3) using the ViCELL XR automated cell counter (Figure 2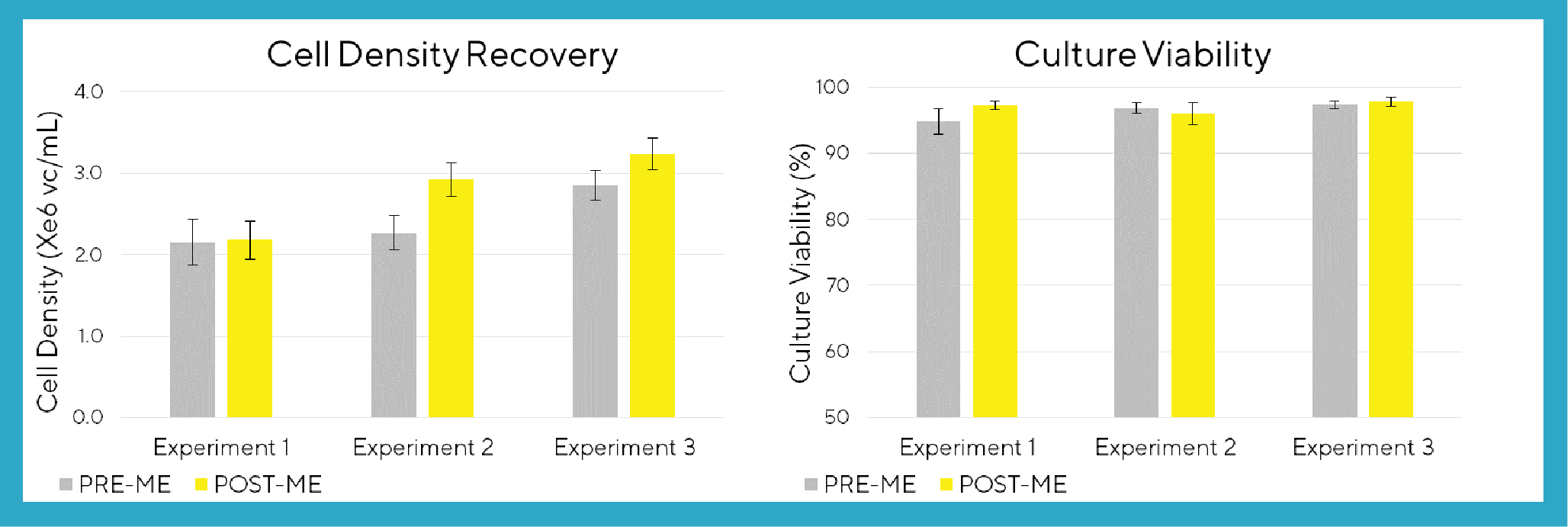
Efficiency of LVV production with medium exchange process 1.0 and proxess 1.5
Titer and transfection efficiency data from Process 1.0 and Process 1.5 (Figure 3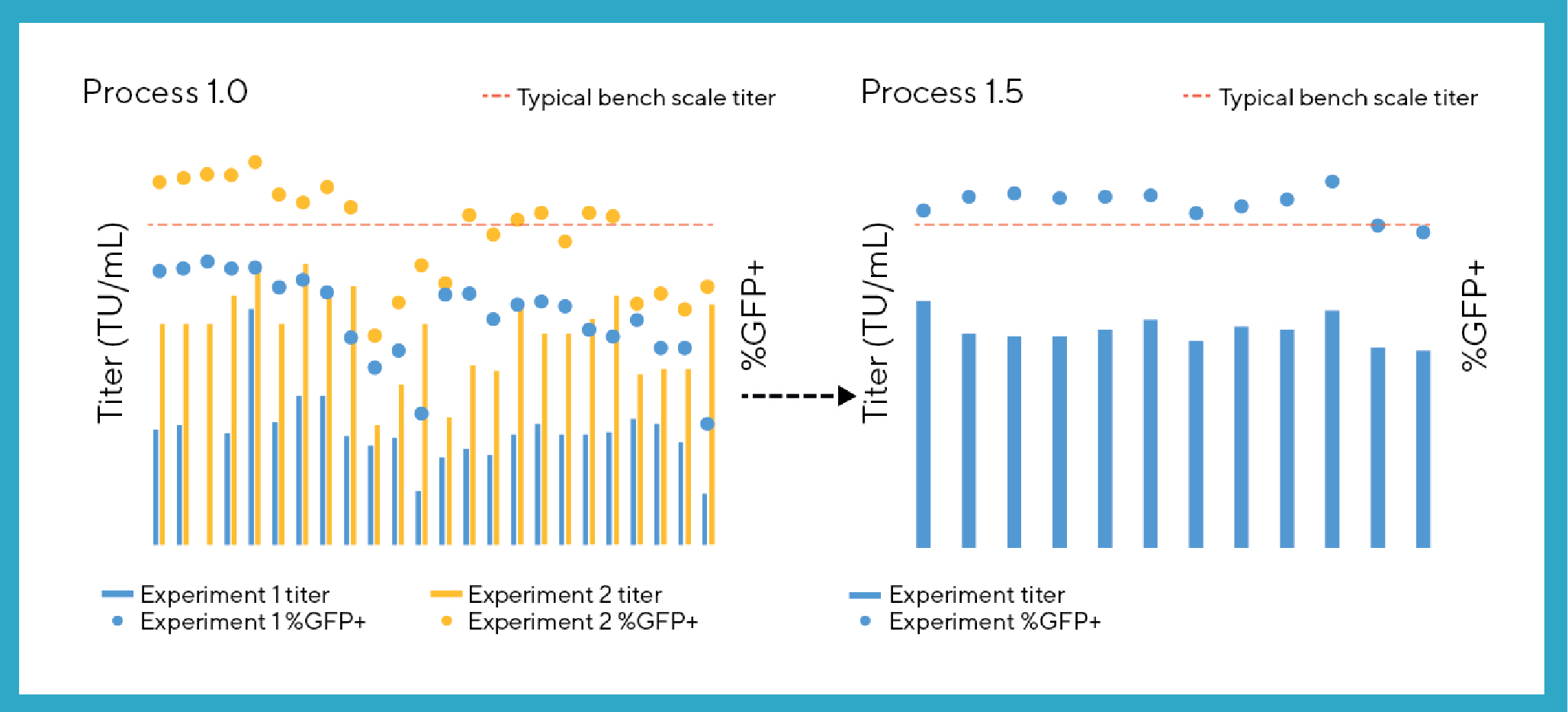
When titer data from replicates are averaged within a study where titers vary across conditions, Process 1.5 demonstrates well defined and reproducible trends for this output (data not shown). However, there were still instances of variability between replicate vessels observed, albeit at low frequency. This allowed for the identification of trends in productivity during development exercises but required the use of replicates or triplicates for each condition during the runs.
Efficiency of LVV production with medium exchange process 2.0
A VCD of 2-3 x106cells/mL and cell viability of 95-98 % is consistently achieved across all microbioreactor replicates, as shown by Figure 3. These results indicate that VCD and cell viability are not compromised by the automated cell settling method used in Process 2.0 for medium exchange.
Furthermore, the average LVV titer achieved when using the Process 2.0 cell settling method for medium exchange in 10 Ambr® 15 microbioreactors was comparable to the average titer achieved using an established proprietary cell culture and medium exchange process run at bluebird bio in 22 bench scale bioreactors (Figure 4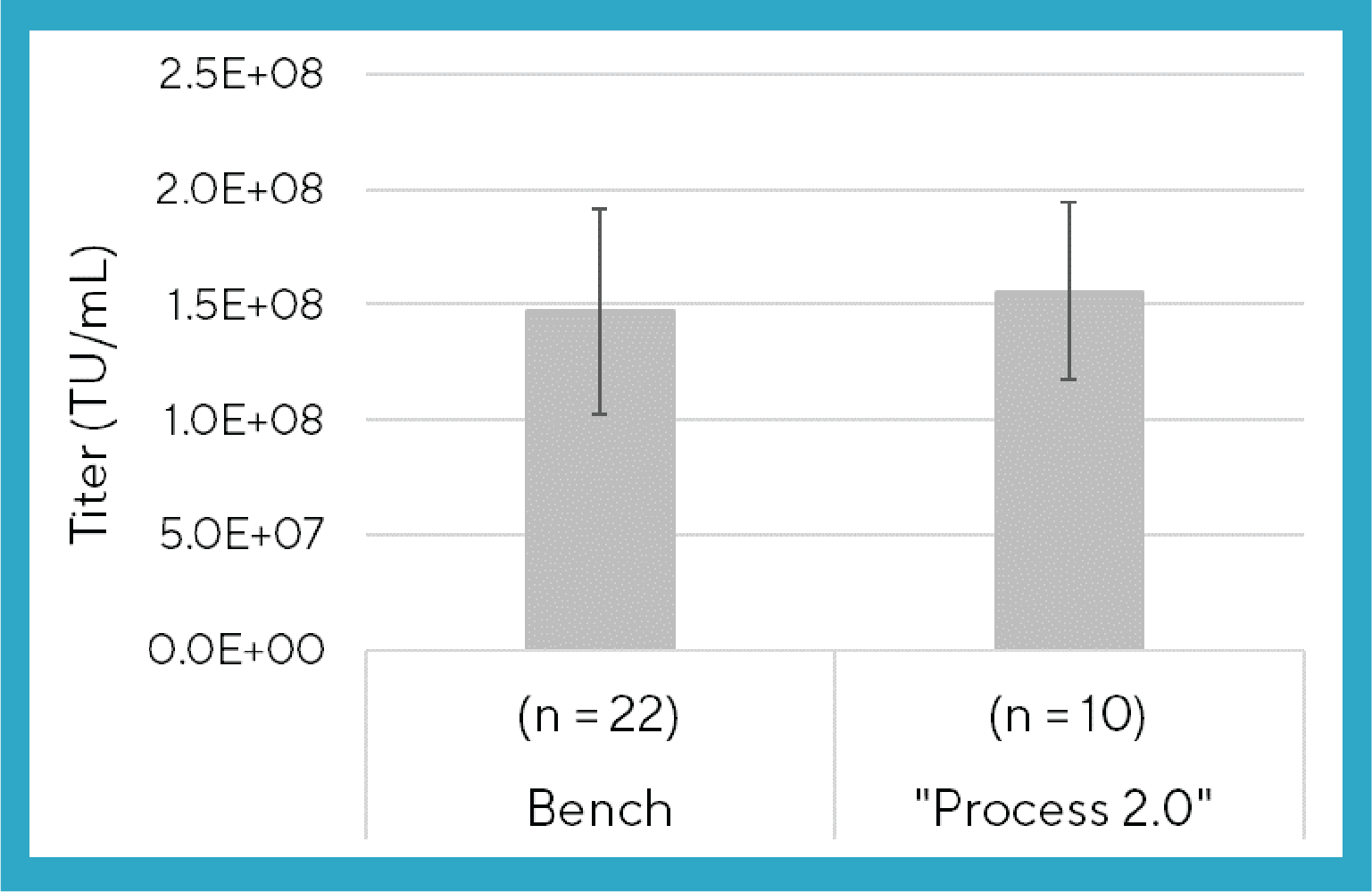
Comparative analysis of LVV production using different medium exchange protocols
To determine whether the Process 2.0 medium exchange protocol is more robust than Processes 1.0/1.5 in terms of LVV yield predictability, the viral particle titer (p24 amount in ng/mL) and the transfection efficiency (%GFP+) from all three processes were plotted and analyzed statistically using an ordinary least squares (OLS) regression analysis. The results from the two methods of measuring process performance, which both correlate to infectious titer, have a positive correlation with Process 2.0 with a R2 value of 0.64 versus little correlation (R2 value of 0.0021) with Processes 1.0/1.5 (Figure 5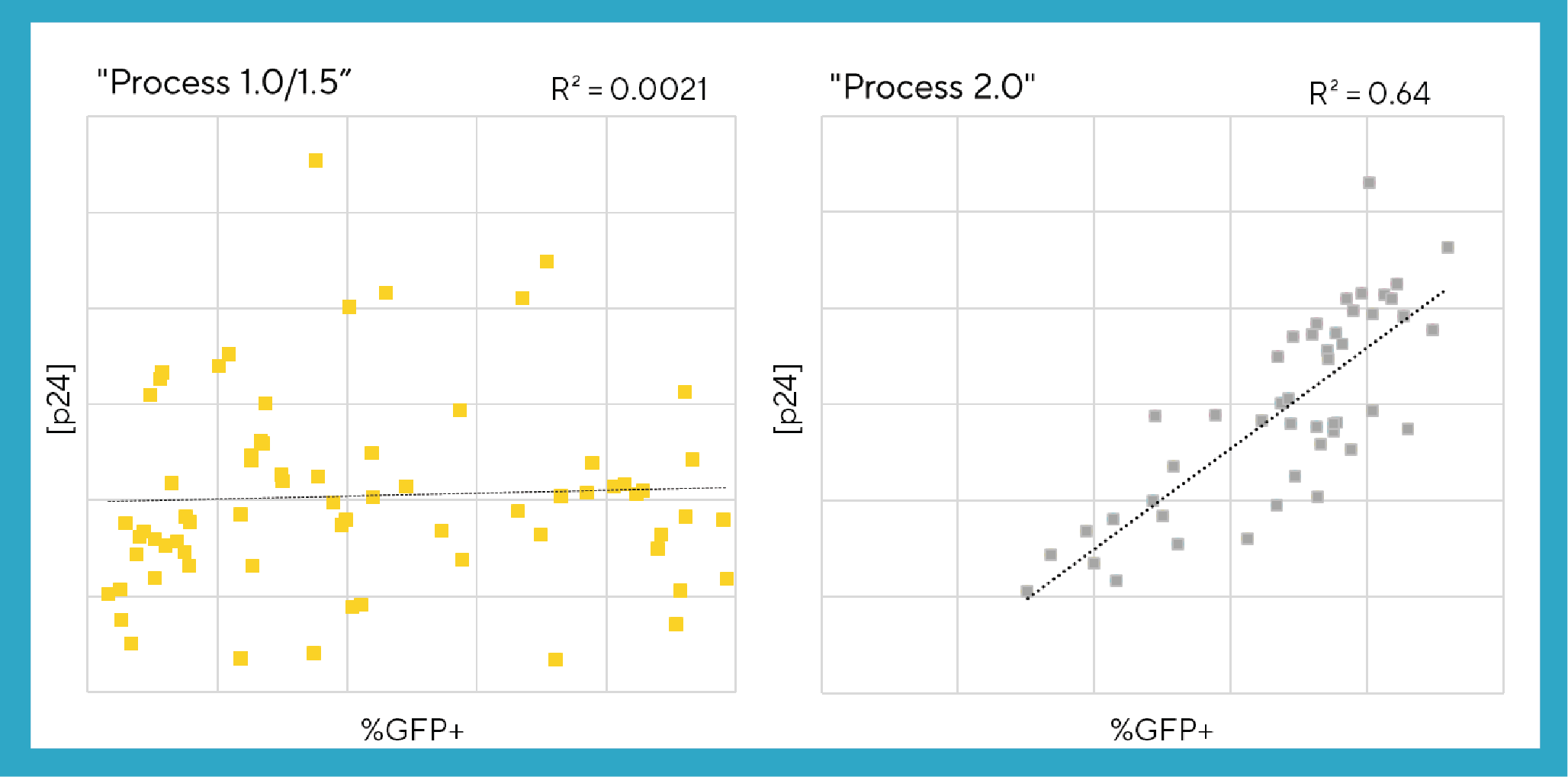
Conclusion
In this article, the development of a medium exchange protocol for a LVV production process using an Ambr® 15 cell culture system has been described. The process developed focused on the medium exchange step, as this process step cannot be scaled-down from a bench-scale STR due to system differences. However, by adjusting to system differences and developing novel process steps and techniques through trialing three different medium exchange protocols, LVV titer and transfection data showed that the Ambr® 15 system can meet the productivity of bench top bioreactors in terms of infectious titer. As the Ambr® 15 bioreactors utilize smaller volumes of media (10–15 mL) than benchtop bioreactors they also have the potential to increase screening capacity, while making costs savings when performing this type of screening.
From the three medium exchange processes assessed, the automated cell settling step, followed by spent media removal and replenishment used in Process 2.0, produced the lowest variability between microbioreactor vessels and improved overall LVV production. The implementation of Process 2.0 also produced results which closely aligned with LVV yield from an established bench-scale process. In summary this study, demonstrates the automation power of the Ambr® 15 cell culture system and its suitability as a process screening tool which can significantly reduce operator variability and handling time with process development of LVV production in suspension culture.
Refernces
1. Anguela XM, High KA. Entering the Modern Era of Gene Therapy. Annu. Rev. Med. 2019; 70, 273–288. https://doi.org/10.1146/annurev-med-012017-043332 Crossref
2. Clinicaltrials.gOv Database Search for Clinical Trials Using Lentiviral Vectors. Crossref
https://clinicaltrials.gov/ct2/results cond=lentiviral+vector+OR+lentivirus+OR+LVV&Search=Apply&recrs=b&recrs=a&recrs=f&recrs=d&age_v=&gndr=&type=&rslt=
3. FDA press release: FDA approval brings first gene therapy to the United States Crossref
https://www.fda.gov/news-events/press-announcements/fda-approval-brings-first-gene-therapy-united-states
4. Kymriah cost dispute goes to heart of pharma’s pricing row. Thepharmaletter 2018. Crossref
https://www.thepharmaletter.com/article/novartis-overpricing-kymriah-by-nearly-200-says-journal-report
5. Comisela R-M, Kara B, Frederick H. Fiesser FH, Farida SS. Lentiviral vector bioprocess economics for cell and gene therapy commercialization.Biochemical Engineering Journal. 2021; 167: 107868. Crossref
https://doi.org/10.1016/j.bej.2020.107868
6. Martínez-Molina E, Chocarro-Wrona C, Martínez-Moreno D, Marchal JA, Boulaiz H. Large-Scale Production of Lentiviral Vectors: Current Perspectives and Challenges. Pharmaceutics. 2020; 12(11):1051. https://doi.org/10.3390/pharmaceutics12111051 Crossref
7. Macchiarulo E, Bassett P, Dudley M. Challenges and progress in lentiviral vector bioprocessing. Cell Gene Therapy Insights. 2018; 4 (10): 915–925. https://doi.org/10.18609/cgti.2018.091 Crossref
8. Lewis G. et al. Novel Automated Micro-Scale Bioreactor Technology: A Qualitative and Quantitative Mimic for Early Process Development. BioProcess J. 2010; 9(1): 22–25. http://dx.doi.org/10.12665/J91.Wales Crossref
9. Moses S, et al. Assessment of AmbrTM as a model for high-throughput cell culture process development strategy. Advances in Bioscience and Biotechnology, 2012; 3: 918–927. https://doi.org/10.4236/abb.2012.37113 Crossref
10. Hsu WT, et al. Advanced microscale bioreactor system: a representative scale-down model for bench-top bioreactors. Cytotechnology 2012; 64(6): 667–78. Crossref
https://doi.org/10.1007/s10616-012-9446-1
11. Lange I et al. Reduced Timelines in Early Process Development. Bioprocess International 2014;10: 34–37. Crossref
https://bioprocessintl.com/wp-content/uploads/2014/11/12-10-LangeVV-Secured.pdf
12. Rees-Manley A, Evaluation of a Small-Scale Perfusion Mimic for Intensified Processes. Genetic Engineering & Biotechnology News 2019; 39 (12): 52–54 Crossref
https://doi.org/10.1089/gen.39.12.14
13. Ransome I, Implementation of Single-Use Miniature Bioreactors to Support Intensified Cell Culture. BioProcess International. 2020; 18 (3): 42–45. Crossref
https://bioprocessintl.com/wp-content/uploads/2020/04/18-3-Sartorius-Ian-Ransome-et-al.pdf
14. Tuvesson O, Uhe C, Rozkov A, Lüllau E. Development of a generic transient transfection process at 100 L scale. Cytotechnology. 2008; 56 (2):123–36. Crossref
https://doi.org/10.1007/s10616-008-9135-2
15. Boucher E. et al. Qualification of a p24 ELISA Assay for Quantitation of Total Lentiviral Vector Concentration. Molecular Therapy. 2016; 24, (1) S187 Crossref
https://doi.org/10.1016/S1525-0016(16)33282-8
Affiliations
Nolan Sutherland,
Scientist, bluebird bio, Inc
Lesley Chan,
Associate Director of Cellular Process Development, bluebird bio, Inc
Kelly Kral,
CMC Lead, bluebird bio, Inc
Franziska Bollmann,
Process Technology Consultant Viral-based Therapeutics, Sartorius Stedim Biotech GmbH
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: The authors would like to acknowledge Lara Nascimento-Brooks, Market Entry Strategy Manager at Sartorius, for helpful revision of this manuscript. The authors would also like to thank Ryan Cassidy and Jesse Milling for their scientific and technical advice on the manuscript.
Disclosure and potential conflicts of interest: F Bollman is an employee of Sartorius. N Sutherland is an employee of and holds stock in bluebird bio. The authors have no other conflicts of interest to disclose.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2021 Archibald P & Shillings A. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: May 25 2021; Revised manuscript received: Sep 13 2021; Publication date: Sep 23 2021.
