Scalability comparison between 50 and 500 liter stirred tank bioreactor for production of rAAV viral vector
Cell & Gene Therapy Insights 2021; 7(9), 1025–1033
10.18609/cgti.2021.131
Viral vectors are a new class of biologics which facilitate gene transfer and modification in living cells, potentially treating a multitude of conditions with genetic causes. Scalable manufacturing technologies are critical to ensuring these cutting-edge medicines can be produced in sufficient quantities to meet the needs of process development, clinical trials, and ultimately commercial manufacturing [1]Ayuso E. Manufacturing of recombinant adeno-associated viral vectors: new technologies are welcome. Mol. Ther. 2016; 3: 15049.Ayuso E. Manufacturing of recombinant adeno-associated viral vectors: new technologies are welcome. Mol. Ther. 2016; 3: 15049.Ayuso E. Manufacturing of recombinant adeno-associated viral vectors: new technologies are welcome. Mol. Ther. 2016; 3: 15049.. As viral vector-based products have only relatively recently received regulatory approval, public information on scalable optimization of these processes is very limited. Abeona Therapeutics is a gene therapy company developing novel gene replacement therapies for rare inherited diseases. These conditions can impact development and limit both quality of life and/or life expectancy [2]Lloyd A, Piglowska N, Ciulla T et al. Estimation of impact of RPE65-mediated inherited retinal disease on quality of life and the potential benefits of gene therapy. Br. J. Ophthalmol. 2019; 103(11): 1610–14. . These transformative medicines can be used to replace a defective gene with a functional copy, silence a defective gene or even directly edit genes [3]Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019; 18(5): 358–78. , [4]Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM. Gene therapy vectors based on adeno-associated virus type 1. J. Virol. 1999; 73(5): 3994–4003. . We evaluated the Pall Allegro™ STR bioreactor family as an rAAV vector production platform and evaluated the scalability of the PEI-mediated transfection manufacturing process for rAAV at the 50 L and 500 L working volume. Process scalability was evaluated based on cell growth, metabolic profile, and vector production. This testing demonstrates that control of key process parameters enables a scalable vector production process between the 50 L and 500 L scale using Allegro STR single use bioreactors.
Viral vectors based on AAV are the vector of choice for many gene delivery applications [1]Ayuso E. Manufacturing of recombinant adeno-associated viral vectors: new technologies are welcome. Mol. Ther. 2016; 3: 15049.Ayuso E. Manufacturing of recombinant adeno-associated viral vectors: new technologies are welcome. Mol. Ther. 2016; 3: 15049.Ayuso E. Manufacturing of recombinant adeno-associated viral vectors: new technologies are welcome. Mol. Ther. 2016; 3: 15049.. These vectors are recombinantly produced virus-like particles (VLPs) based on a type of non-pathogenic parvovirus called adeno-associated virus. These vectors deliver the therapeutic gene to the patient or isolated patient cells [5]Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 1998; 72(3): 2224–32. .
One of the most common production methods used for rAAV production is transient transfection of cultured human cells [6]Powers AD, Piras BA, Clark RK, Lockey TD, Meagher MM. Development and Optimization of AAV hFIX Particles by Transient Transfection in an iCELLis(®) Fixed-Bed Bioreactor. Hum. Gene Ther. Methods 2016; 27(3): 112–21. . This process relies on the introduction of several types of plasmid DNA into the cells to induce vector production. Transfection complex is produced by combining negatively charged DNA and positively charged transfection agent. These two reagents interact to form small particles of transfection complex with a neutral charge that can be introduced into the cell culture vessel or bioreactor and absorbed by the cells. Once the cells recover, they start expressing the viral genes and produce and package the vector.
These manufacturing processes are dependent on time consuming procedures and expensive raw materials [1]Ayuso E. Manufacturing of recombinant adeno-associated viral vectors: new technologies are welcome. Mol. Ther. 2016; 3: 15049.Ayuso E. Manufacturing of recombinant adeno-associated viral vectors: new technologies are welcome. Mol. Ther. 2016; 3: 15049.Ayuso E. Manufacturing of recombinant adeno-associated viral vectors: new technologies are welcome. Mol. Ther. 2016; 3: 15049.. Scalable manufacturing processes are critical to providing the quantities of vector needed to bring these potentially life-saving treatments to waiting patient populations. Many gene therapy manufacturing processes rely on culturing HEK293 cell lines (or derivative AAV293 cell lines), and several early and current forms of production culture these cells on an adherent substrate [7]Robert MA, Chahal PS, Audy A, Kamen A, Gilbert R, Gaillet B. Manufacturing of recombinant adeno-associated viruses using mammalian expression platforms. Biotechnol. J. 2017; 12(3). Robert MA, Chahal PS, Audy A, Kamen A, Gilbert R, Gaillet B. Manufacturing of recombinant adeno-associated viruses using mammalian expression platforms. Biotechnol. J. 2017; 12(3). .
Here we evaluate the performance of an rAAV transient transfection production process in the 50 L and 500 L Allegro™ STR bioreactors (Figure 1
| Table 1 Equipment and materials used in the study. | ||
| Equipment | Manufacturer | Model/part no. |
| Allegro STR 50 Bioreactor | Pall Corporation | STR 50-JC110-R-SU |
| LAUDA Integral T 2200 | LAUDA | L002242 |
| Allegro STR 500 Bioreactor | Pall Corporation | STR 500-JC110 |
| LAUDA VC 10000 | LAUDA | S190003372 |
| Nova Flex 2 Bioanalyzer | Nova Biomedical | T08310040 |
| Vi-Cell XR | Beckman Coulter | 30527950 |
| pH probe InPro3253/225/pt1000 | Mettler Toledo | 52200966 |
| DO probe InPro 6800/12/220 | Mettler Toledo | 52002569 |
| Allegro 50 L Biocontainer | Pall Corporation | 6412-0927L |
| Allegro 500 L Biocontainer | Pall Corporation | X6412-0891S |
| Materials | Manufacturer | Model/part/serial no. |
| Suspension AAV293 Master Cell Bank | Abeona | N/A |
| FreeStyle F17 media | Thermo Fisher | A1383504 |
| GlutaMAX 100X | Thermo Fisher | 35050061 |
| 100X/10% Pluronic F-68 | Thermo Fisher | 24040032 |
| DENERASE, 5MU | c-LECTA | 20804-5M |
| PEIpro | PolyPlus Transfection | #115-100 |
| pHelp, pAAV, pGOI Plasmids | Aldevron | |
Materials & methods (Table 1)
Seed Train
Suspension-adapted AAV293 cells were thawed from cryopreservation and cultivated in a 125 mL shake flask (30 mL working volume) using Freestyle™ F17 media supplemented with 4 mM GlutaMAX™ (Thermo Fisher). Growth and incubator conditions are shown in Table 2. One day post-vial thaw, the culture was expanded to a 250 mL flask at 60 mL working volume. Viable cell density and viability were monitored using a Vicell™ XR (Beckman). Cells were maintained between 0.2 and 2.0 x 106 cells/mL during cell expansion. The culture volume was scaled-up a total of 6 passages until three 3 L flasks were used to inoculate an Allegro STR 50 bioreactor at a working volume of 20 L.
| Table 2 Shake flask seed train parameters. | |
| Parameter | Target |
| Media | FreeStyle F17 + 4 mM GlutaMAX |
| Incubator CO2 setpoint (%) | 5 |
| Incubator temperature (°C) | 37 |
| Incubator humidity (%) | Ambient with water reservoir |
| Shaker speed for flask sizes < 3 L (rpm) | 120 |
| Shaker speed for 3 L shake flasks (rpm) | 72 |
| Shaker orbit (mm) | 19 |
N-1 bioreactor
The Allegro STR 50 bioreactor was used as the N-1 bioreactor by inoculating at a density of 0.2 x 106 cells/mL at a 20 L initial volume and expanded to 50 L two days later. A summary of the N-1 culture process parameters is shown in Table 3. The viable cell density was adjusted daily as the growth rate was slightly higher than anticipated. This was done to prevent the cells from growing above 2.0 x 106 cells/mL for transfection on a specific pre-planned day.
| Table 3 N-1 process parameters. | ||
| Parameter | STR 50 (20 L working volume) | STR 50 (50 L working volume) |
| Basal medium | FreeStyle F17 + 4 mM GlutaMAX + 0.1 % Pluronic F-68 | |
| Working volume (L) | 20 | 50 |
| Power input P/V (W/m3) | 30 | |
| Agitation (rpm) | 65 | 88 |
| Air sparge flowrate (L/min) | 0.1 | |
| Overlay flowrate (L/min) | 0.2 | |
| pH | Pre-conditioned with 10% CO2 – no active control post-inoculation | |
| Dissolved oxygen (%) | 40 | |
| Temperature (°C) | 37 | |
| Inoculation cell concentration | 0.2 x 106 cells/mL | |
Bioreactor production
The same inoculation strategy was used for production in the STR 500 as the N-1 passage in the STR 50. A uniform inoculation pool of 275 L was prepared in the STR 500. 25 L was then transferred to a new STR 50 vessel. This resulted in both vessels inoculated at half capacity with the same cell density. The vessels were then expanded to the full working volume after 24 hours. The culture was continued for 2 days until the viable cell density (VCD) reached the target transfection density of ∼1.0 x 106 cells/mL.
The process control parameters and PID settings are shown in Tables 4 & 5.
| Table 4 Production bioreactor operating parameters. | ||||
| Parameter | STR 500 (PRE feed-up) | STR 500 (POST feed-up) | STR 50 (PRE feed-up) | STR 50 (POST feed-up) |
| Basal medium | FreeStyle F17 + 4mM GlutaMAX + 0.1 % Pluronic F-68 | |||
| Initial working volume (L) | 250 | 475 | 25 | 47.5 |
| Power input P/V (W/m3) | 30 | |||
| Agitation (RPM) | 72 | 90 | 70 | 88 |
| Agitation direction | Downflow | |||
| Air flowrate (L/min) | 0.5 | 1.0 | 0.1 | 0.1 |
| Overlay flowrate (L/min) | 2.0 | 2.0 | 0.2 | 0.2 |
| pH | Pre-conditioned with 10% CO2 – no active control post-inoculation | |||
| Dissolved oxygen (%) | 40 | |||
| Temperature (°C) | 37 | |||
| Initial cell density | 0.2 x 106 cells/mL | |||
| Table 5 PID settings for production. | |||||
| Parameters | Setpoint | P | I | D | Dead-band |
| DO | 40 | 5 | 500 | 0 | 0 |
| Temperature | 37 | 20 | 500 | 0 | N/A |
The process parameter scaling strategy utilized for this comparison is to scale-up based on constant power per unit volume and normalized gas flow. Because of minor geometry difference between the impellers of the two vessels, similar power per unit volume outputs are achieved using slightly different rotational rates.
Transfection complex was prepared by diluting appropriate amounts of each plasmid DNA and PEIPro™ into cell culture media, combining the reagents and allowing the mixture to incubate for 15 minutes before addition to the bioreactors. This process was performed separately for each bioreactor.
DENARASE™ (30 U/mL, c-LECTA) was added to the bioreactor 24 hours post transfection. The culture was then continued with daily monitoring and harvested when the viability fell to <20%.
A summary of the production process conditions is shown in Table 6 below.
| Table 6 Transfection and DENARASE parameters. | |
| Parameter | Target |
| VCD at transfection (106 cells/mL) | 1.0 |
| Target time of addition completely added post-mix (minutes) | 15 |
| Target DENARASE addition timing (hours post transfection) | 24 |
| Target DENARASE activity in culture (U/mL) | 30 |
| Target amount of media to dilute DENARASE in for STR (mL/L) | 1 |
| Media used for DENARASE addition | F17 + 4 mM GlutaMAX |
Analytical methods
Aliquots were collected for vector production analysis starting 7 days post-transfection. Samples were centrifuged at 500 g for 5 min and clarified supernatant was stored at -20°C.
Viral vector physical titer was measured using droplet digital polymerase chain reaction (ddPCR) assay using the Biorad QX200 droplet System with the Auto DG droplet generator. The PCR primer/probe (IDT) combination targeted an amplicon contained in the gene of interest of the rAAV transfer genome.
Results
N-1 bioreactor
Suspension adapted AAV293 cells were recovered from cryopreservation as described in the methods section. The culture was expanded for 6 passages before sufficient biomass was generated to inoculate the N-1 bioreactor at 40% capacity.
After 2 days of culture, cells were removed, and the volume was adjusted to 100% capacity. Minor volume adjustments (media addition and culture removal) were performed each day to compensate for slightly faster than anticipated cell growth. The faster growth rate observed in the STR 50 than shake flask may have been a result of the bioreactor providing a better controlled environment.
Very good growth and viability were observed in the N-1 culture. The VCD and viability trends are shown in Figure 2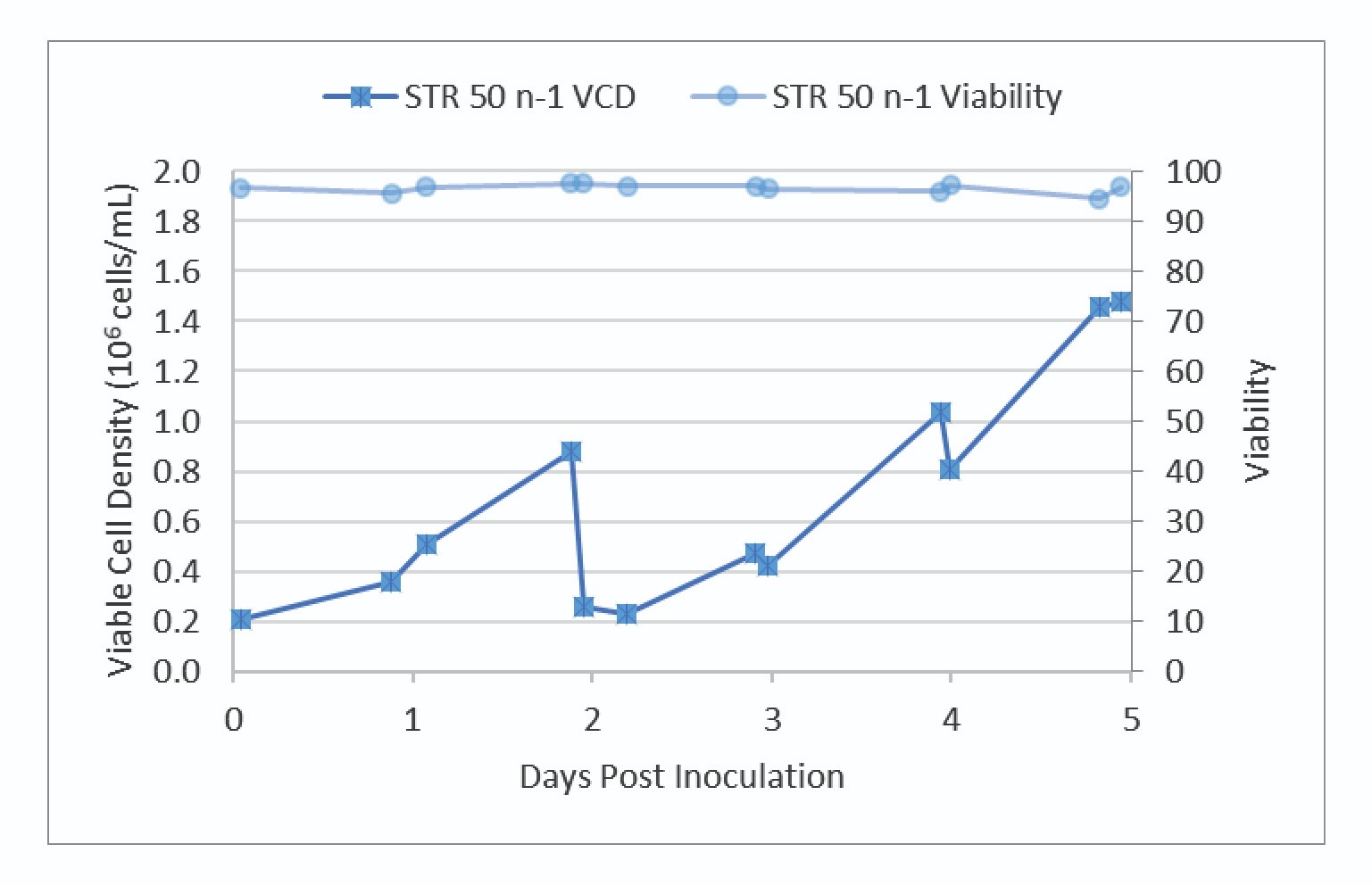
The N-1 bioreactor was harvested on day 5 to inoculate the production STR 500 and control vessel, STR 50.
Production bioreactor
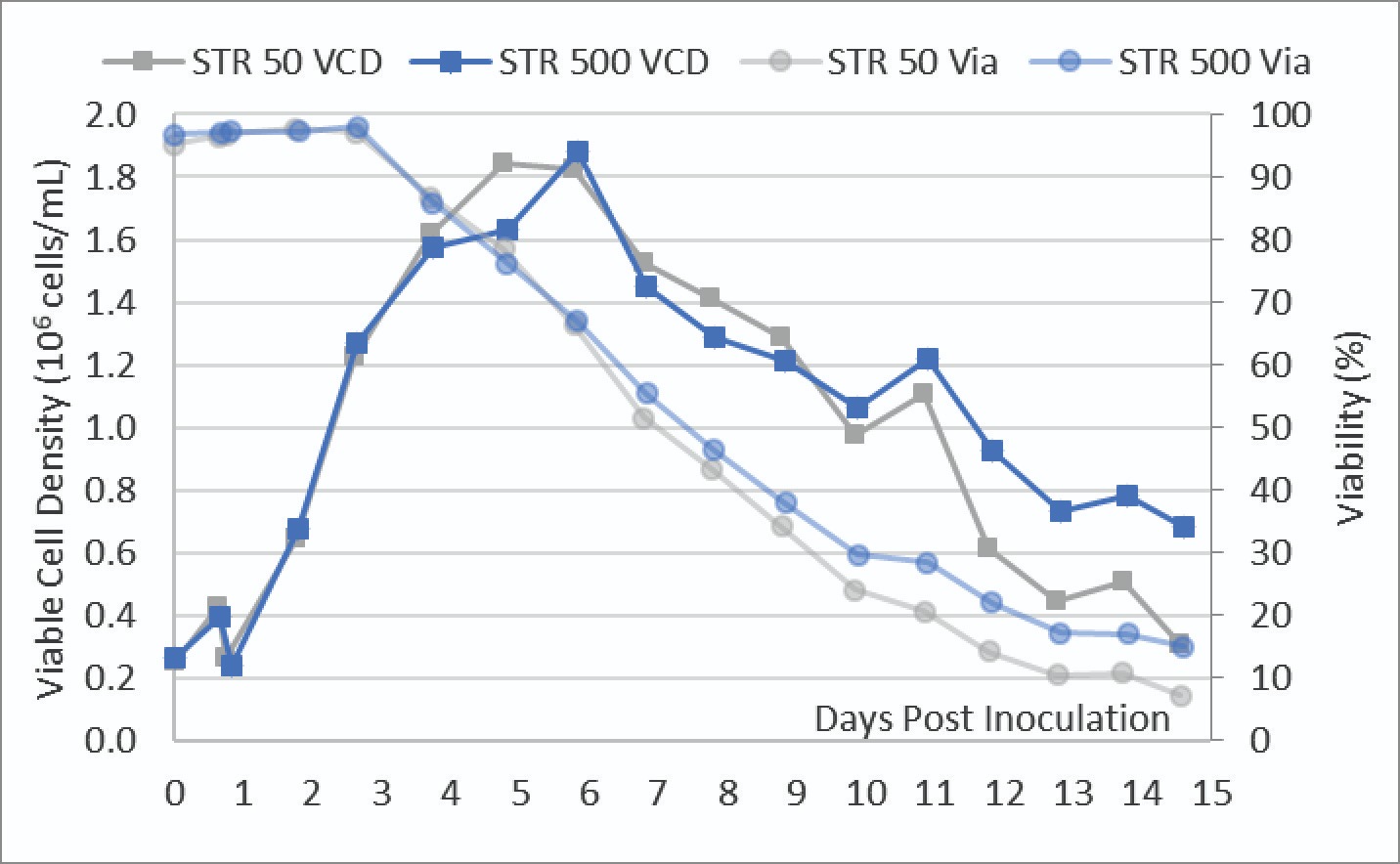
As described in the methods section, a parallel STR 500 and STR 50 were inoculated with a uniform cell culture bolus. The VCD and viabilities of these cultures were measured daily and shown in Figure 3.
These trendlines show near identical cell growth and viability between both the STR 50 and STR 500 cultures up until transfection on day 3. After transfection, there is a drop in viability between the two vessels while the viable cell density continued to increase. Both cultures reached a maximum viable cell density of ∼1.8 x 106 cells/mL. The STR 50 culture showed a slightly lower viability in the second half of the culture.
Nutrient and metabolite analyses were also performed daily. The glucose and lactate profile of the cultures are shown in Figure 4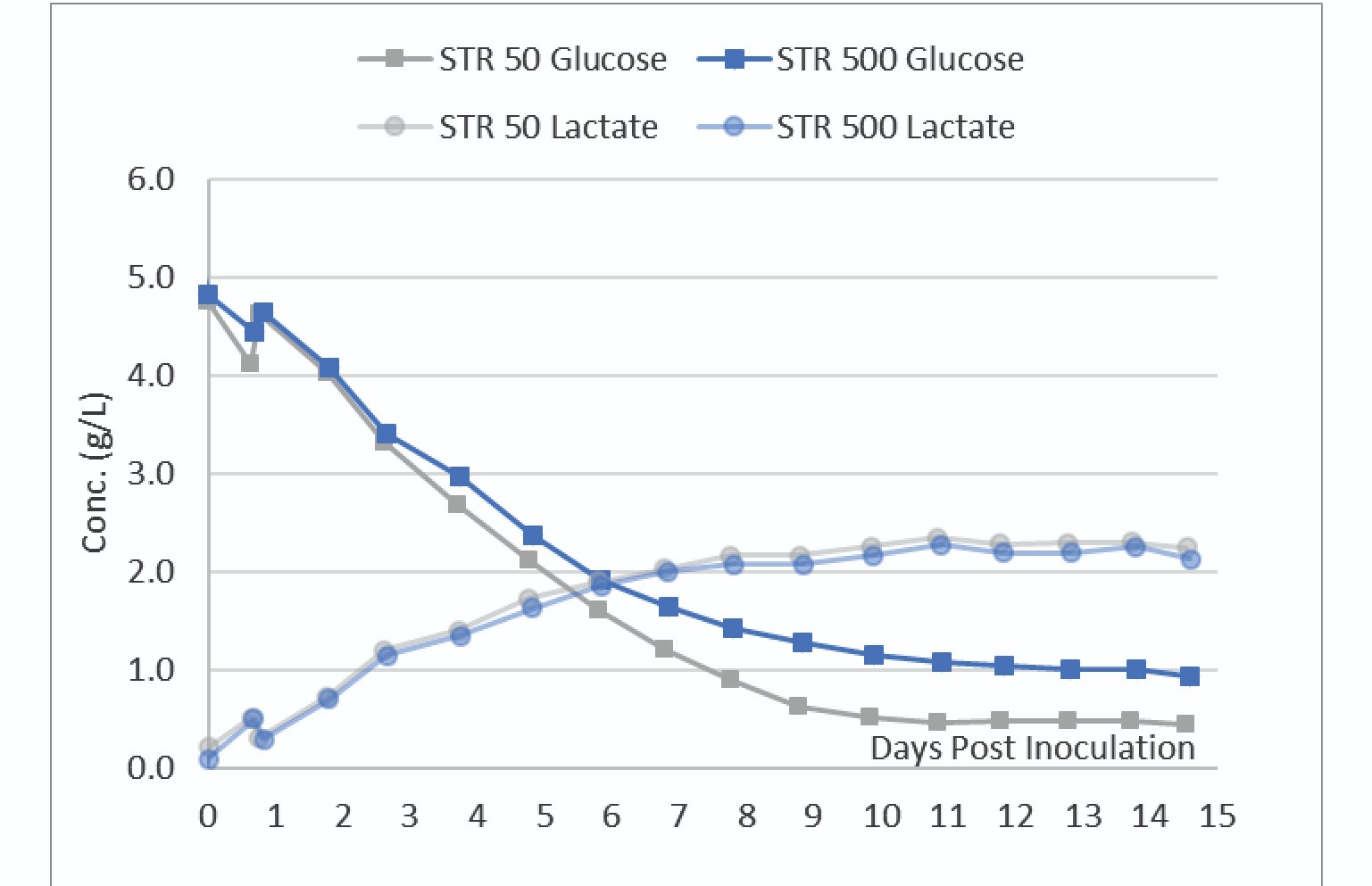
The data in Figure 4 shows the STR 50 consumed slightly more glucose than the STR 500. The VCD data in Figure 3 indicates a slightly higher viable biomass in the STR 500. Slight differences in transfection efficiency between the two vessels could explain these slight differences in VCD and metabolic profiles.
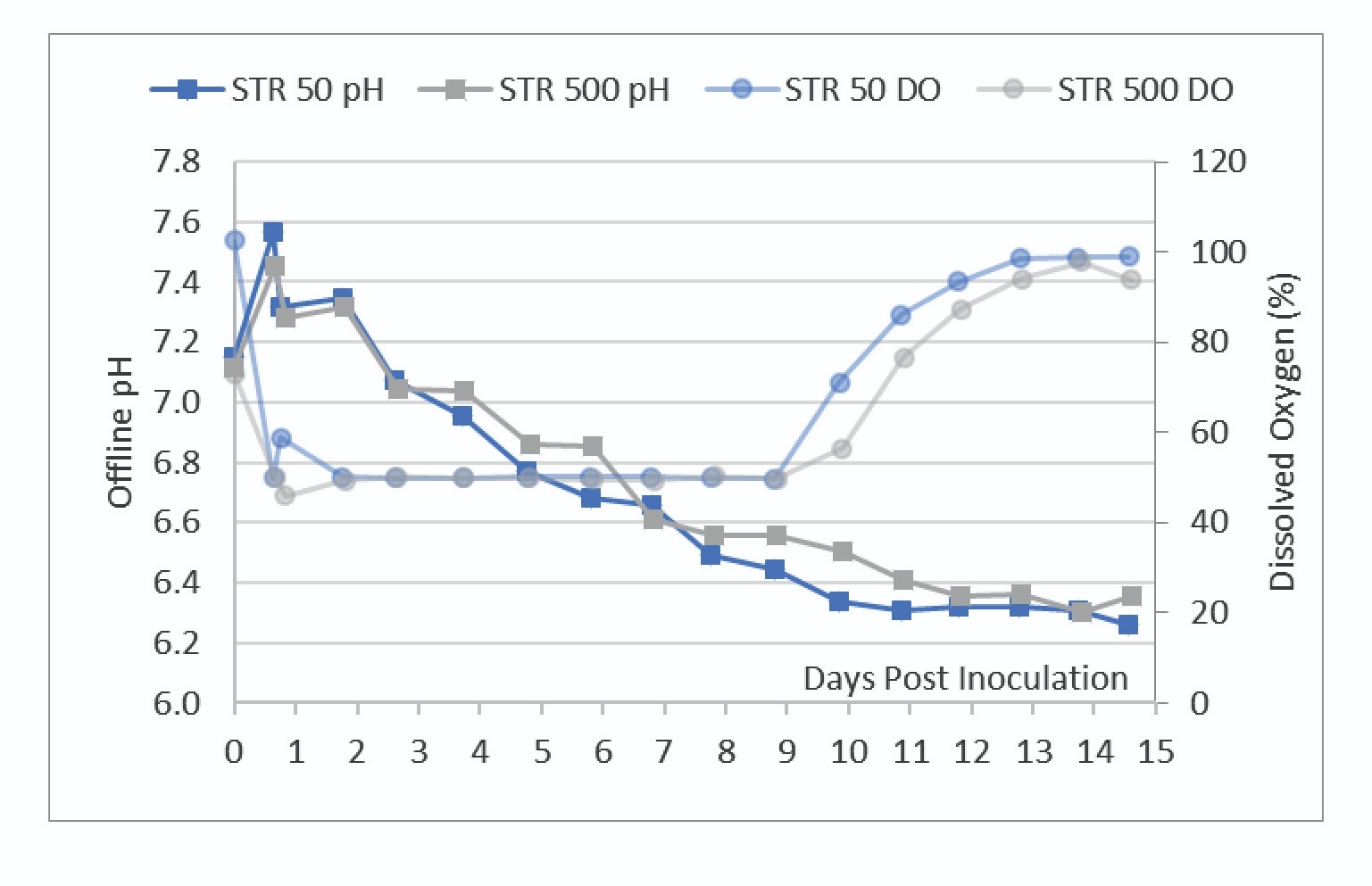
After initial bioreactor conditioning and inoculation, there was no active pH control of the vessels. The pH at both scales trended together, however the STR 50 had slightly lower pH throughout the run. There were no noticeable differences between pCO2 levels between the two scales indicating that the aeration strategy was effective at maintaining a similar bioreactor environment (data not shown).
The DO trendlines shown in Figure 5 showed the bioreactors were able to maintain one-side DO control at the 50% setpoint.
To maintain control of the DO at this setpoint, the bioreactor adjusts the O2 sparge rate. Figure 6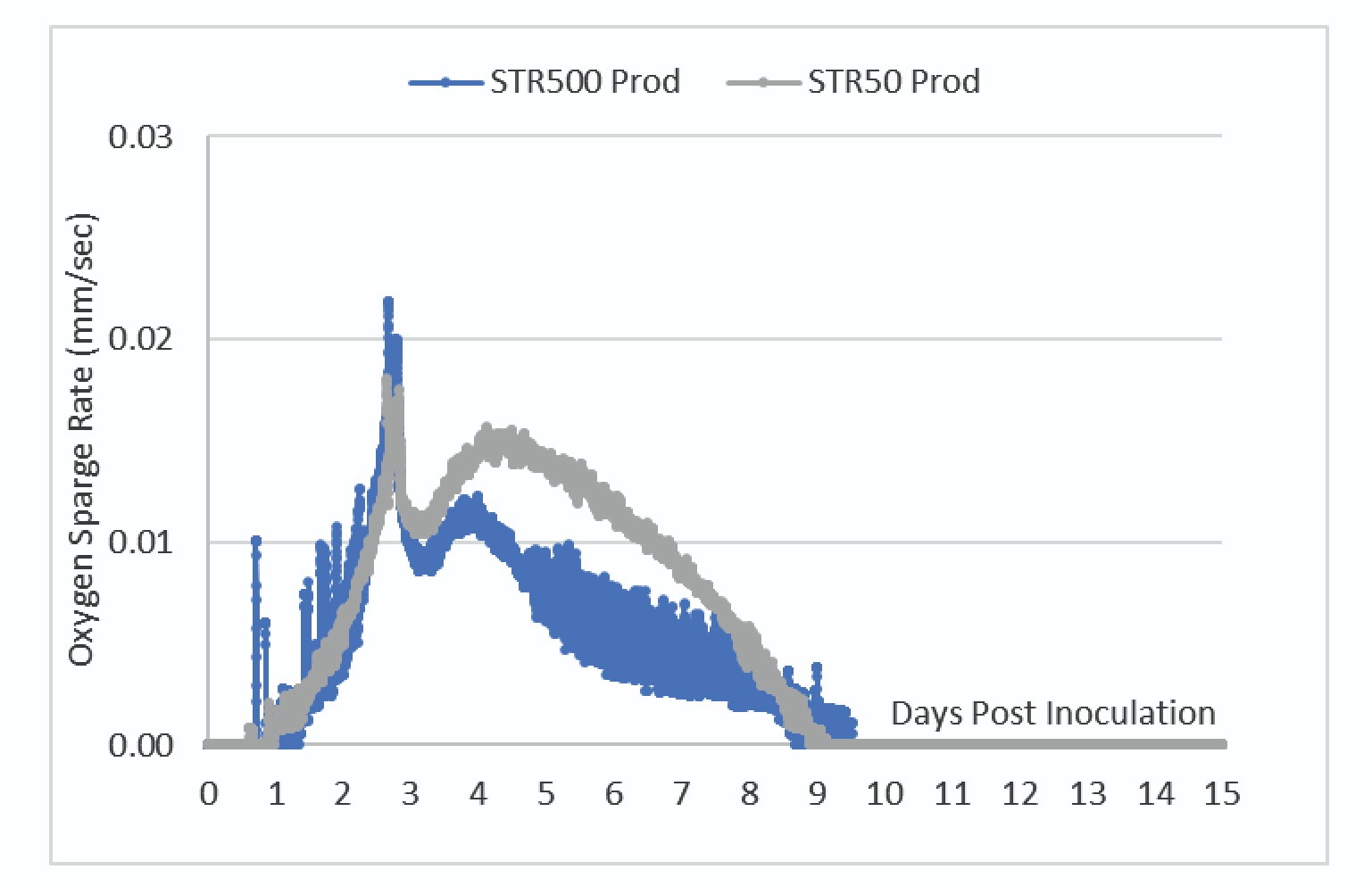
When the culture was analyzed for product titer, the productivity between the two scales was within 10–20% with slightly higher titers observed in the STR 50. The titer data collected during the run is shown in Figure 7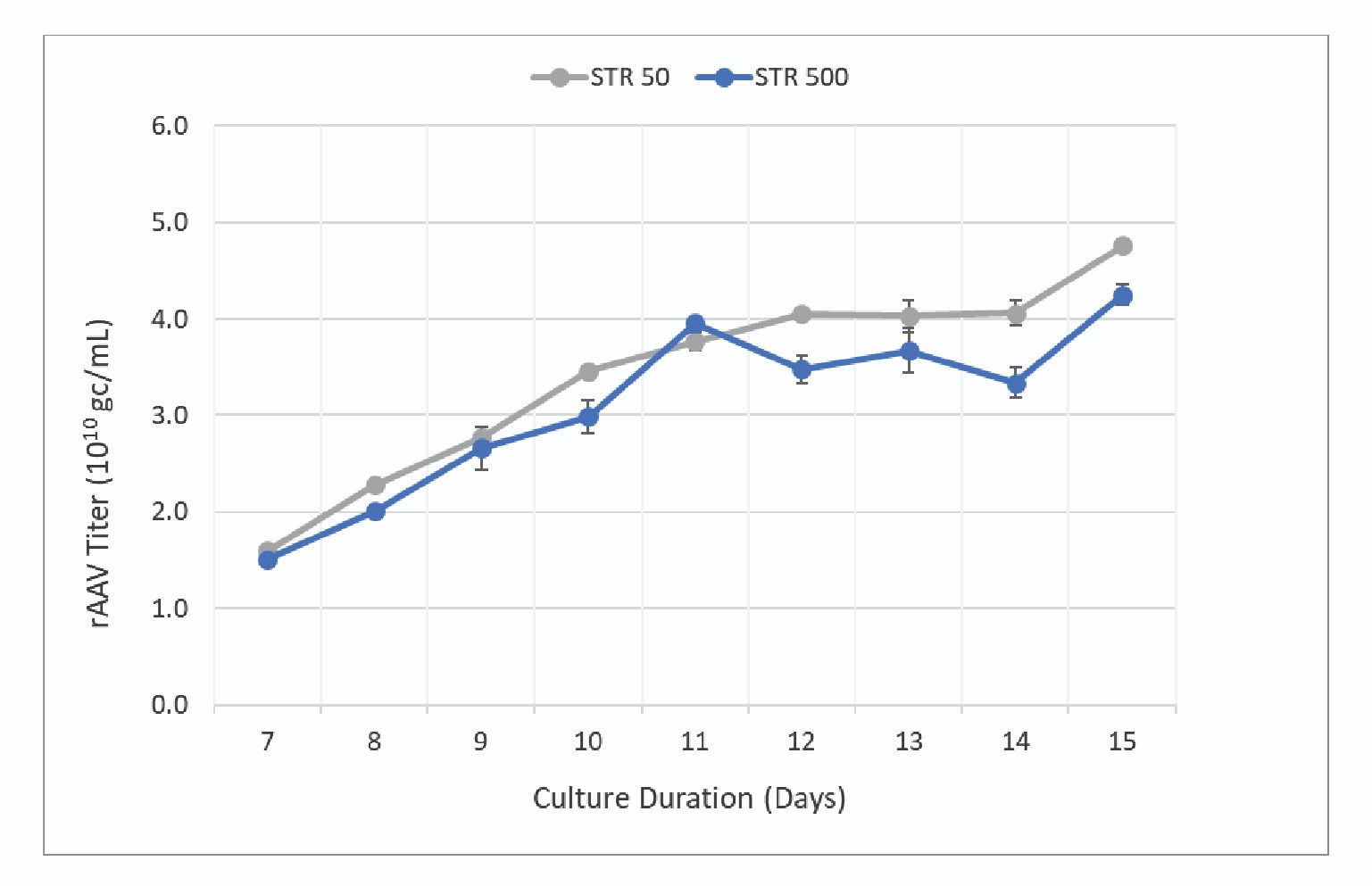
The data shows rAAV titer increases throughout the culture with maximum titer being observed at harvest. Final product titers were 4.3 x 1010 gc/mL and 4.8 x 1010 gc/mL for the STR 500 and STR 50 respectively. Error bars represent standard deviation of 8 replicates (2 assay dilutions with 4 technical replicates each). The two cultures had final harvest titers within ∼10% indicating a scalable process.
Conclusions/Discussion
Scalable upstream technologies are critical to enable the manufacturing capacity needed to bring gene therapy treatments with large patient populations to market. Optimal bioreactor performance can be achieved when the bioreactor is able to provide a controlled, uniform environment so that each cell can realize its full productivity potential.
The data presented here demonstrates that the Allegro STR 50 and STR 500 bioreactors are appropriate for rAAV production and that they produced similar bioreactor environments at both the 50 L and 500 L scales. Some minor differences in metabolic profile were observed after transfection. The root cause was not identified but was likely related to slightly different efficiencies of plasmid transfection.
This scalable bioreactor performance resulted in cultures with similar growth profiles, viability and viral vector productivity. This scalability is realized when utilizing Pall’s recommended scale up strategy across the Allegro STR family
Translational Insights
This work demonstrates scalability of this transfection-based production process between the 50 L and 500 L scale. Production at the 500 L scale is critical to providing sufficient vector for clinical trials and may be sufficient for full manufacturing capacity for certain indications, but for many others, further scale-up to 1,000 L and 2,000 L will be required.
Pall’s single-use bioreactors are available up to 2,000 L. There are a number of other technologies in the industry which are being utilized to further increase vector productivity. Improvements to expression systems with improved packaging efficiency and development of producer cell lines are a couple technologies being evaluated to further increase vector yields [7]Robert MA, Chahal PS, Audy A, Kamen A, Gilbert R, Gaillet B. Manufacturing of recombinant adeno-associated viruses using mammalian expression platforms. Biotechnol. J. 2017; 12(3). Robert MA, Chahal PS, Audy A, Kamen A, Gilbert R, Gaillet B. Manufacturing of recombinant adeno-associated viruses using mammalian expression platforms. Biotechnol. J. 2017; 12(3). .
References
1. Ayuso E. Manufacturing of recombinant adeno-associated viral vectors: new technologies are welcome. Mol. Ther. 2016; 3: 15049. Crossref
2. Lloyd A, Piglowska N, Ciulla T et al. Estimation of impact of RPE65-mediated inherited retinal disease on quality of life and the potential benefits of gene therapy. Br. J. Ophthalmol. 2019; 103(11): 1610–14. Crossref
3. Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019; 18(5): 358–78. Crossref
4. Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM. Gene therapy vectors based on adeno-associated virus type 1. J. Virol. 1999; 73(5): 3994–4003. Crossref
5. Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 1998; 72(3): 2224–32. Crossref
6. Powers AD, Piras BA, Clark RK, Lockey TD, Meagher MM. Development and Optimization of AAV hFIX Particles by Transient Transfection in an iCELLis(®) Fixed-Bed Bioreactor. Hum. Gene Ther. Methods 2016; 27(3): 112–21. Crossref
7. Robert MA, Chahal PS, Audy A, Kamen A, Gilbert R, Gaillet B. Manufacturing of recombinant adeno-associated viruses using mammalian expression platforms. Biotechnol. J. 2017; 12(3). Crossref
Affiliations
Todd P Sanderson
Pall Corporation; Westborough MA, USA
Timothy Erlandson
Pall Corporation; Westborough MA, USA
Nathan Hazi
Pall Corporation; Westborough MA, USA
Anne MacIntyre
Pall Corporation; Westborough MA, USA
Benjamin I Ingersoll
Abeona Therapeutics; Cleveland, OH, USA
Michael McLaughlin
Abeona Therapeutics; Cleveland, OH, USA
Steven Wesel
Abeona Therapeutics; Cleveland, OH, USA
Phillip B Maples
Abeona Therapeutics; Cleveland, OH, USA
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: The authors disclose that Pall Corporation owns patents relevant to the Biotech Industry. Dr Hazi has received travel support from Abeona Therapeutics to carry out STR 50 bioreactor training. Dr McLaughlin and Dr Maples have received support from Abeona Therapeutics. Dr Wesel has received support from Abeona Therapeutics and Pall; and his current employer, Forge Biologics, is a CDMO that manufactures AAV vectors.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2021 Pall Corporation. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Aug 18 2021; Revised manuscript received: Sep 8 2021; Publication date: Sep 24 2021.

