Magnetic selection for consistent cellular starting material in autologous cell therapy manufacture
Cell & Gene Therapy Insights 2022; 8(1), 97–112
10.18609/cgti.2022.027
An efficient cell selection method is crucial to deliver consistent autologous therapy products when the starting material received is highly variable. Whilst a variety of technologies are being adopted in the industry, there are few GMP-compliant options, and these technologies are often manual, semi-automated, and lack commercial viability. In this article, two experts share insights on obtaining highly purified cells using magnetically active cell selection in a flexible, closed manufacturing system. In addition, transitioning manual cell therapy production to scalable automated processes is discussed.
The current state of CAR T cell therapy
Since their development in 1989, chimeric antigen receptor (CAR) T cells have evolved greatly, with second- & third-generation CAR T cells incorporating one or two co-stimulatory domains respectively, and fourth-generation CAR T cells including a gene inducer that drives the production of immune effector molecules such as cytokines and chemokines.
CD19 CAR T cell therapy has been shown as an effective treatment for relapsed/refractory diffuse large B cell lymphoma, an aggressive form of non-Hodgkin Lymphoma. The survival probability after treatment with CD19 CAR T cells has been shown to be superior to that of conventional chemotherapy for non-Hodgkin lymphoma [1]Neelapu SS, Locke FL, Bartlett NL, et al. Comparison of 2-year outcomes with CAR T cells (ZUMA-1) vs salvage chemotherapy in refractory large B-cell lymphoma. Blood Adv. 2021 Oct 26;5, 4149–4155., [2]Frederick L. Locke, David B et al. Primary Analysis of ZUMA-7: A Phase 3 Randomized Trial of Axicabtagene Ciloleucel (Axi-Cel) Versus Standard-of-Care Therapy in Patients with Relapsed/Refractory Large B-Cell Lymphoma. Blood 2021 138 (Supplement 1), .
There are currently five FDA-licensed CAR T cell therapies on the market, all of which are second-generation CAR T cell therapies, using either a CD28 or 41BB costimulatory domain. However, none of these CAR T cell therapies is currently licensed in New Zealand.
A novel CD19 cell therapy: From China to New Zealand
The Malaghan Institute of Medical Research in Wellington, New Zealand has developed a clinical CAR T cell product containing a third-generation CD19 CAR construct using CD28 and TLR2 costimulatory domains (Figure 1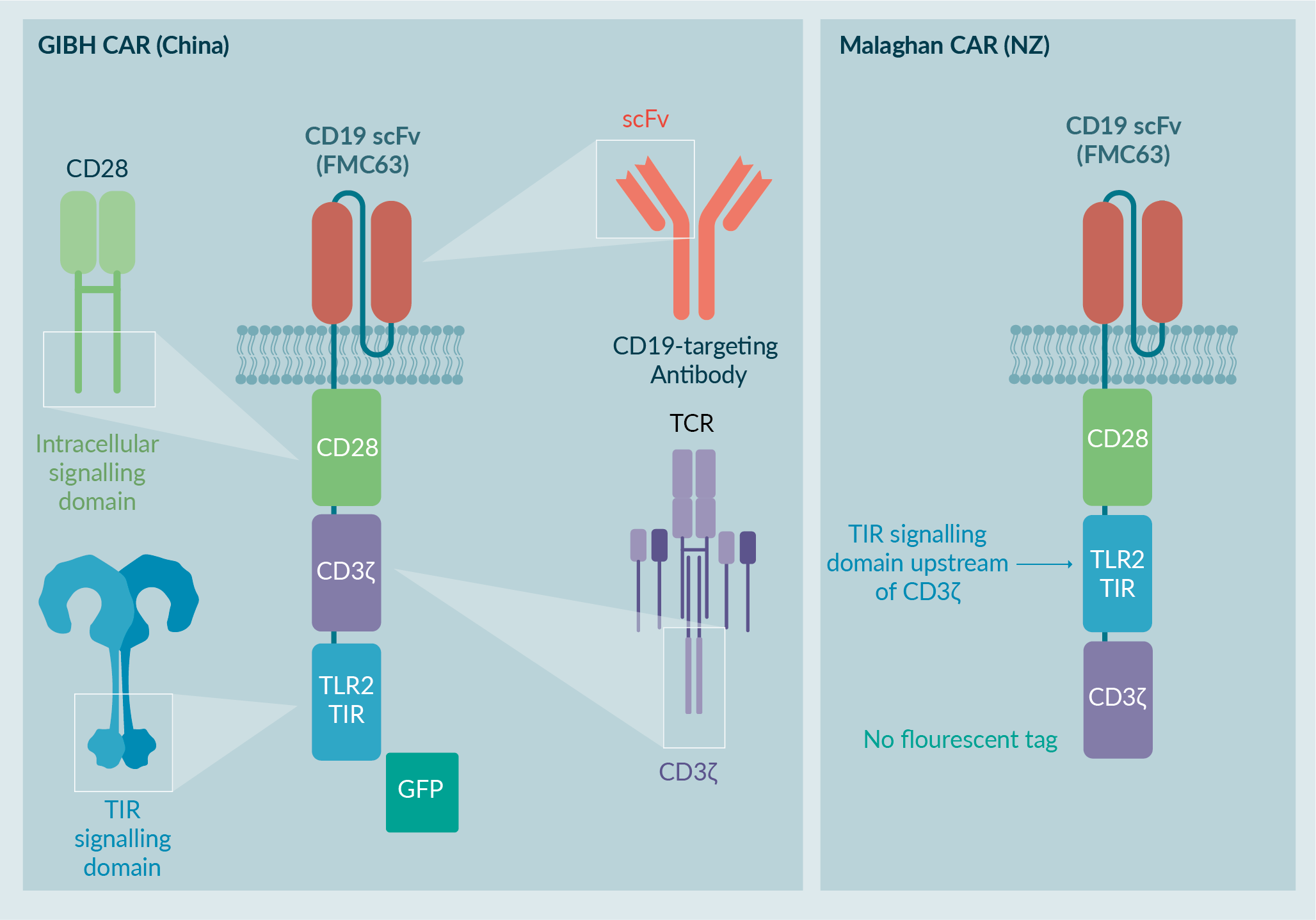
Guangzhou Institutes of Biomedicine & Health Phase 1 CAR T cell clinical trial
The first-in-human trial of these third-generation T cells was conducted in China, as a Phase 1 dose escalation study with split-dose infusion, using 5×104; 5×105; 1×106 CD19-CAR-T2 (1928zT2) T cells/kg. The study treated 29 patients, who were suffering from chemotherapy resistant or refractory CD19+ B cell acute lymphocytic leukemia (B-ALL), with extra-medullary disease [4]Weng J, Lai P, Qin L et al. A novel generation 1928zT2 CAR T cells induce remission in extramedullary relapse of acute lymphoblastic leukemia. J. Hematol. Oncol. 2018, 20;11,25.Weng J, Lai P, Qin L et al. A novel generation 1928zT2 CAR T cells induce remission in extramedullary relapse of acute lymphoblastic leukemia. J. Hematol. Oncol. 2018, 20;11,25..
Peripheral blood mononuclear cells (PBMCs) were harvested, and CAR T cells produced. Following a lymphodepleting chemotherapy regimen, the CAR T cells were infused in three separate escalating doses over three days. Patients were monitored throughout the study. Data from the first three patients show CAR T cell expansion in the blood after infusion (Figure 2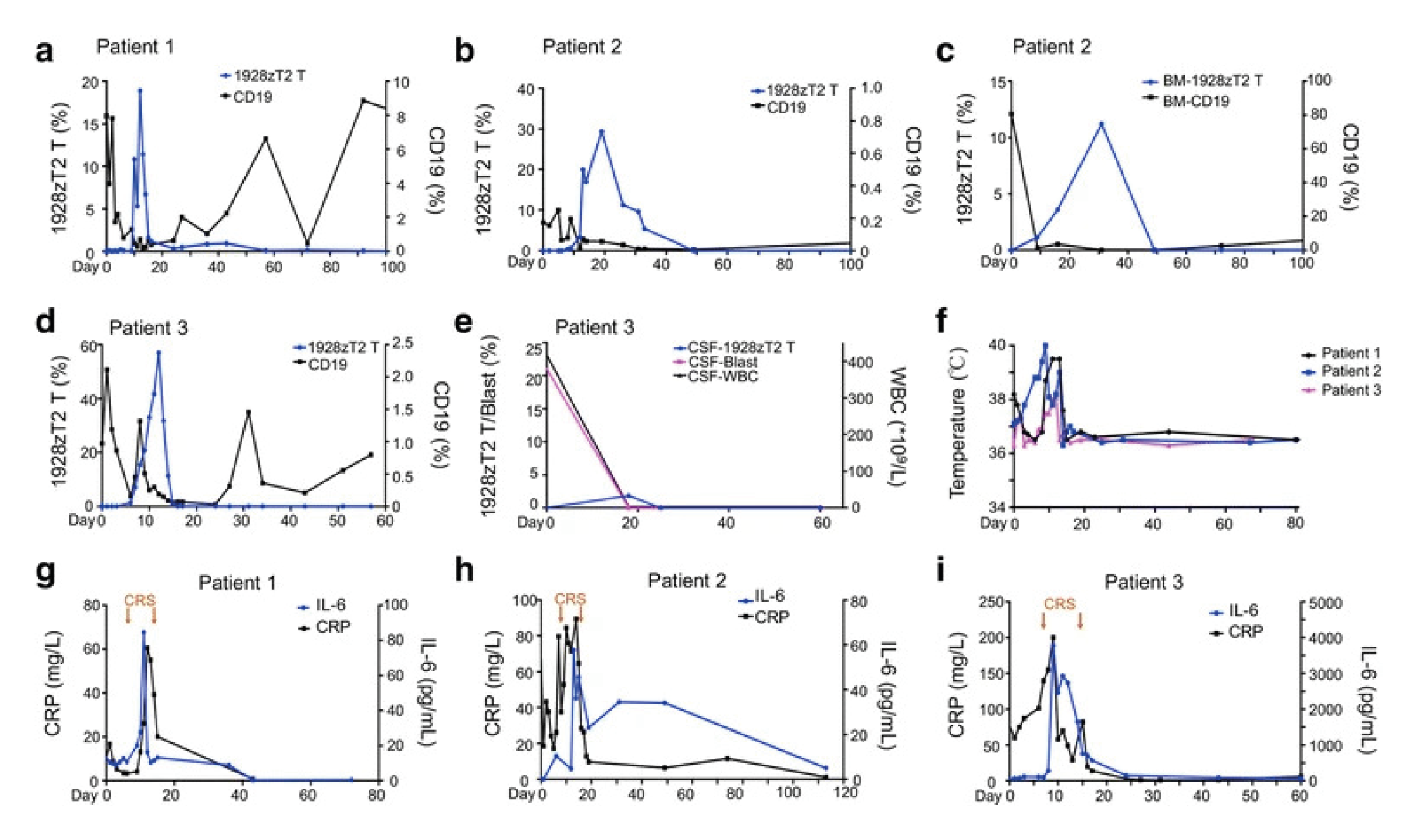
Complete clinical responses were seen at all three dose levels, including resolution of the extra-medullary disease. This included patient 3, who received the highest dose, and had complete resolution of their disseminated disease [4]Weng J, Lai P, Qin L et al. A novel generation 1928zT2 CAR T cells induce remission in extramedullary relapse of acute lymphoblastic leukemia. J. Hematol. Oncol. 2018, 20;11,25.Weng J, Lai P, Qin L et al. A novel generation 1928zT2 CAR T cells induce remission in extramedullary relapse of acute lymphoblastic leukemia. J. Hematol. Oncol. 2018, 20;11,25..
Introducing the TLR2 CAR T cells to the Malaghan Institute of Medical Research clinic
The Malaghan Institute of Medical Research modified the CAR construct to comply with New Zealand regulatory requirements. The fluorescent GFP tag was removed, and the order of the CD3z and TLR2 signaling domains was inverted to resemble other third-generation CAR T cells in clinical trials. The GMP manufacturing process was also adapted to adhere to New Zealand regulatory requirements [5]Weinkove R, George P, Ruka M, Haira TH, Giunti G. Chimeric antigen receptor T-cells in New Zealand: challenges and opportunities. N. Z. Med. J. 2021 Sep 17;134, 96–108..
The Phase 1 CAR T cell clinical trial currently running in New Zealand (ENABLE) is a dose escalation study using 5×104; 1×105; 2×105; 5×105 WZTL-002 (1928T2z) CAR T cells/kg [6]George P, Dasyam N, Giunti G et al. Third-generation anti-CD19 chimeric antigen receptor T-cells incorporating a TLR2 domain for relapsed or refractory B-cell lymphoma: a phase I clinical trial protocol (ENABLE). BMJ Open. 2020;10,e034629.. As of February 2022, 13 patients with relapsed/refractory B cell non-Hodgkin lymphoma have been treated in this study.
Again, PMBCs were harvested and CAR T cells were produced. Patients received lymphodepleting chemotherapy followed by a single dose infusion of CAR T cells. This trial is ongoing, so no patient data is currently available.
Translation of Malaghan’s open, manual manufacturing process into a fully automated workflow
Current manual manufacturing process
The current GMP cell manufacturing process at the Malaghan Institute is fully manual. In a purpose-designed Grade B GMP suite, one or two operators can produce one patient’s CAR T cell product at a time in what is a labor-intensive process.
The manual production process begins with frozen PBMCs, which are thawed and rested overnight, before T cells are selected and activated using magnetic beads. The next day, cells are transduced with a lentiviral vector, the remainder of which is removed by manual media exchange on day 3. On day 4, the magnetic beads are removed. There is further media exchange on days 7 and 9, before harvesting and formulating the cells on day 11.
The manual cell manufacturing process was validated using PBMC from 6 healthy donors, whose CAR T cell products expanded well from both fresh and frozen starting material. Three representatives are shown in Figure 3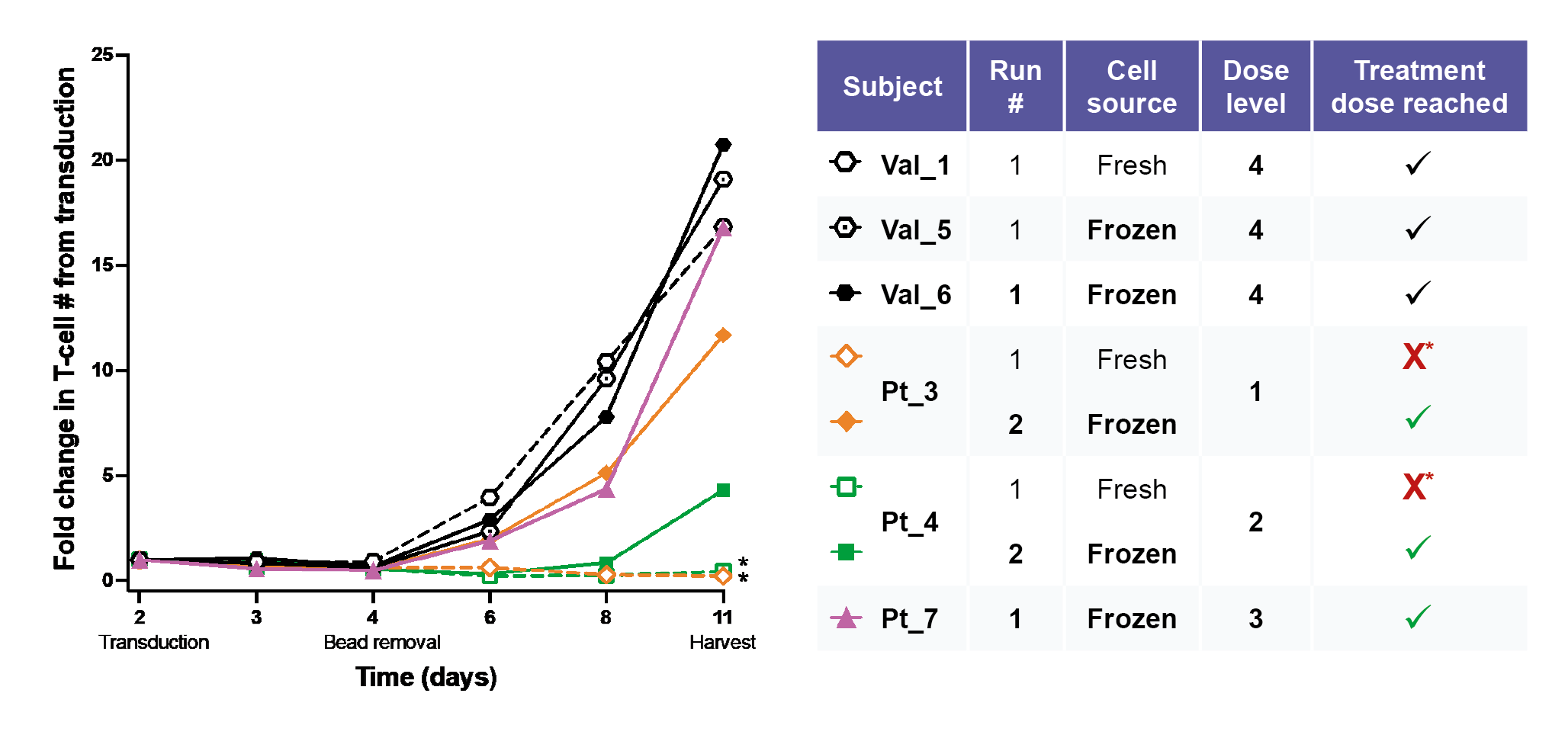
Several further optimizations were made throughout the process, including alterations to plasticware and integrating cell straining steps to remove any dead or clumping cells. After these optimizations, similar expansion rates in patients to the healthy donors were obtained.
Transition to automation to allow for clinical scale-up
Despite this successful optimization, manual cell production has many disadvantages. Only one patient product can be generated at a time due to regulations prohibiting more than one patient’s cells being in open culture in a single laboratory. Major operator intervention is required, leading to a time-consuming process. In addition, as the process is in open culture, sterility testing must be performed at the conclusion of the production run.
Closed system automation allows for the growth of multiple patient products at once in the same space, subject to regulatory approval by MedSafe. Considerably less operator-intervention is required, and in-process testing of sterility and quality is possible.
The Cocoon® Platform method of automation was chosen because it is compatible with the existing GMP reagents, allows for magnetic separation to be integrated into the process, is a single closed system unit, and has a small footprint allowing for potential scale-out. Lonza’s research and development team in Ontario, Canada helped to transfer the manual CAR T cell process to an automated version for use in the Cocoon® Platform.
Although this new process still requires PBMC thaw and rest to be done manually, T cell selection through lentiviral vector transduction, vector removal, bead removal, and media exchange, can all be done in an automated way within the Cocoon® Platform. This requires minimal manual intervention on a few days throughout the process. Formulation after cell harvest is still done manually in the current process, but there are possibilities to automate the PBMC processing and formulation steps in the future.
In terms of CAR T cell expansion, the Cocoon® Platform performs favorably when compared to the manual process (Figure 4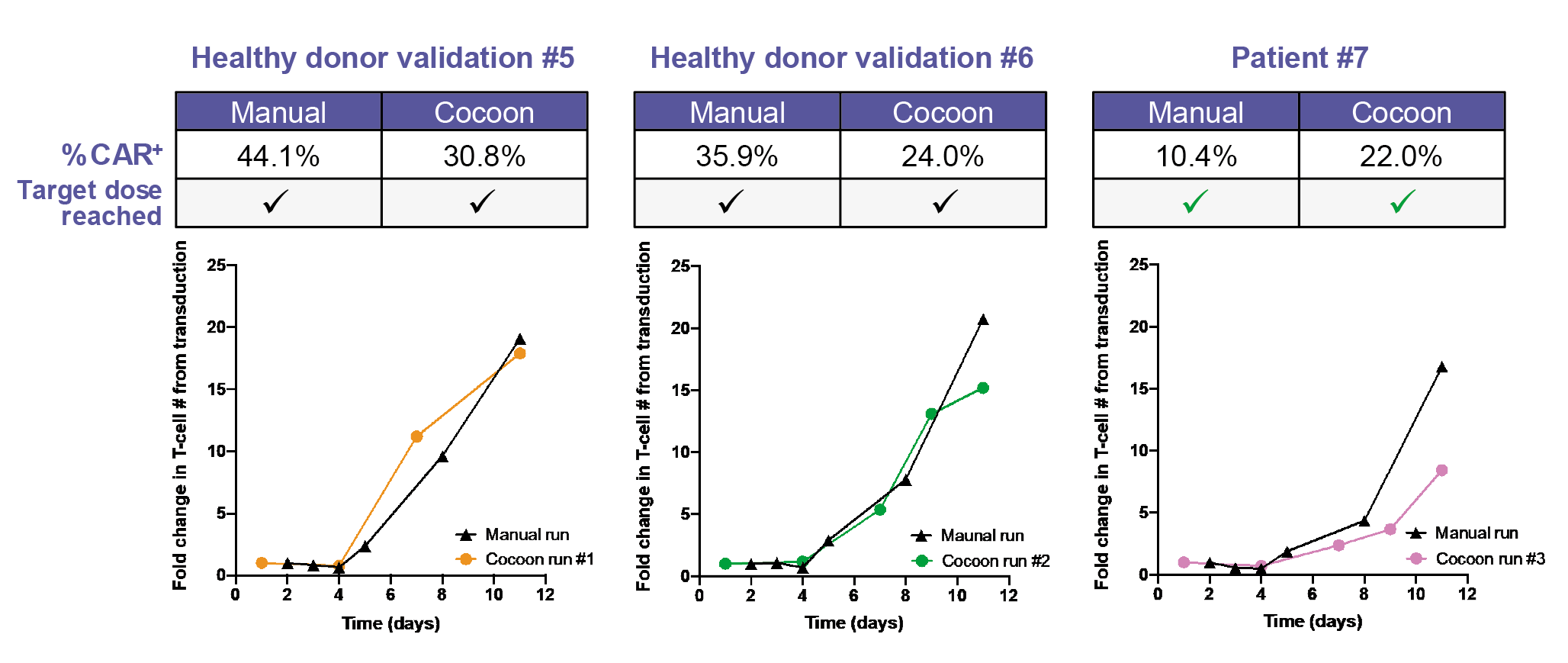
Due to minor variability in CAR T cell percentage, the protocol was further tweaked. Successful production of the treatment dose in both the manual and Cocoon® Platform runs was achieved in patient 7, with the CAR T cell transduction efficiency in the Cocoon® Platform being superior to that of the manual process.
Vision for the future
The Malaghan Institute has formed a joint venture with BioOra to co-fund CAR T cell automation in New Zealand. In the short-term, deployment of Cocoon® Platform-produced cells within an expansion cohort of the ENABLE Phase 1 trial is planned, alongside the implementation of in-process quality testing to speed up the product release process.
In the medium-term, priorities include increasing production capacity without increasing the facility footprint, by running several Cocoon® Platforms in the same space.
Long-term plans involve scale-up and scale-out of CAR T cell production by creating a dedicated manufacturing site run by BioOra, allowing for larger scale deployment of CAR T cells to be used commercially or within clinical trials.
The Cocoon® Platform explained
The Cocoon® Platform is a functionally closed automated system designed to reduce touchpoints, thus reducing human error, increasing reliability, improving product quality, and reducing labor and costs. This system can be used in Grade C cleanrooms, which are anticipated to become more prevalent in the coming years.
The inside chamber within the Cocoon® Platform is divided into a top section maintained at 37°C with controlled CO2 levels, and a bottom section maintained at 4°C where fresh media is safely stored. In the center of the top section is a peristaltic pump, which moves the fluid around inside the cassette. Actuators along the lower portion of the top section open and close fluidic pathways within the cassette. Built-in sensors monitor levels of dissolved oxygen and pH, providing feedback during the process.
Integrated magnetic separation
Integrated magnetic separation is a new feature soon to be introduced for the Cocoon® Platform. This will enable the platform to be capable of fully automating the whole process between cell expansion and final formulation, including bead removal.
There have been two major modifications that allow for the integrated magnetic separation. One is on the new magnetic separation cassette, which has a magnetic separation line where the separation occurs. The second is the addition of an internal magnet, which can be toggled ‘on’ and ‘off’. The magnetic separation Cocoon® Platform is suitable for positive or negative selection, as well as bead removal in-cassette. Multiple bead systems have been tested and additional optimization efforts are ongoing.
Beads are captured inside the magnetic separation line. Once captured and separated from the rest of the sample, the beads can either be kept or discarded as needed. This process is entirely automated inside the cassette and Cocoon® Platform.
Magnetic separation – Cocoon® Platform preliminary data
The Cocoon® Platform can achieve upwards of 95% purity following bead separation, with a viability of around 90%. Around 99% of all beads can be removed by the de-beading process. With a seeding count of approximately 60 million cells, 2 billion cells can be produced by day 10 (a 30-fold expansion) whilst maintaining a viability of 90%.
No significant difference is observed in terms of purity of cells separated using the Cocoon® Platform compared to the control, when using CD3/CD28 Dynabeads (Figure 5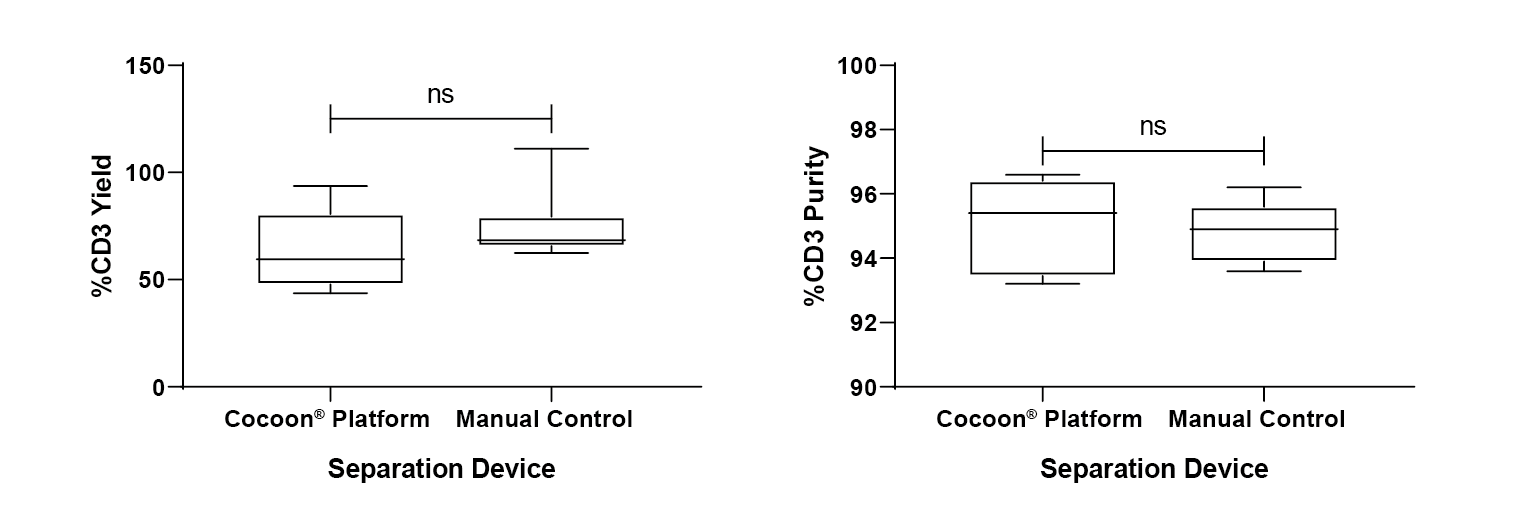
No significant difference can be seen in terms of final cell yield or final viability by day 10 between the Cocoon® Platform and the magnetic separation Cocoon® Platform (Figure 6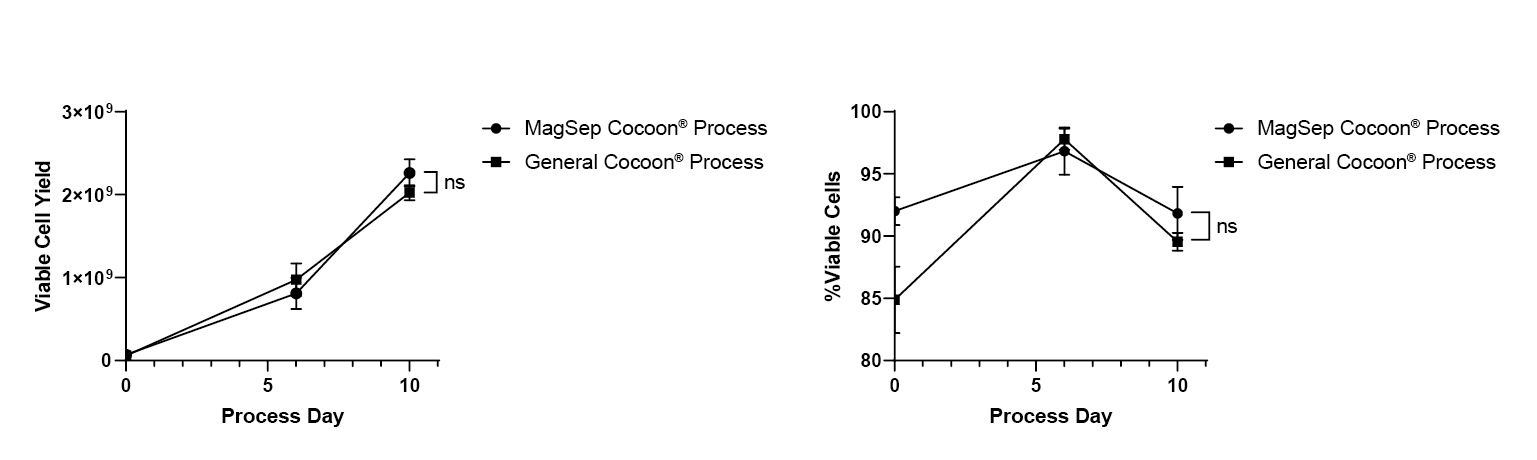
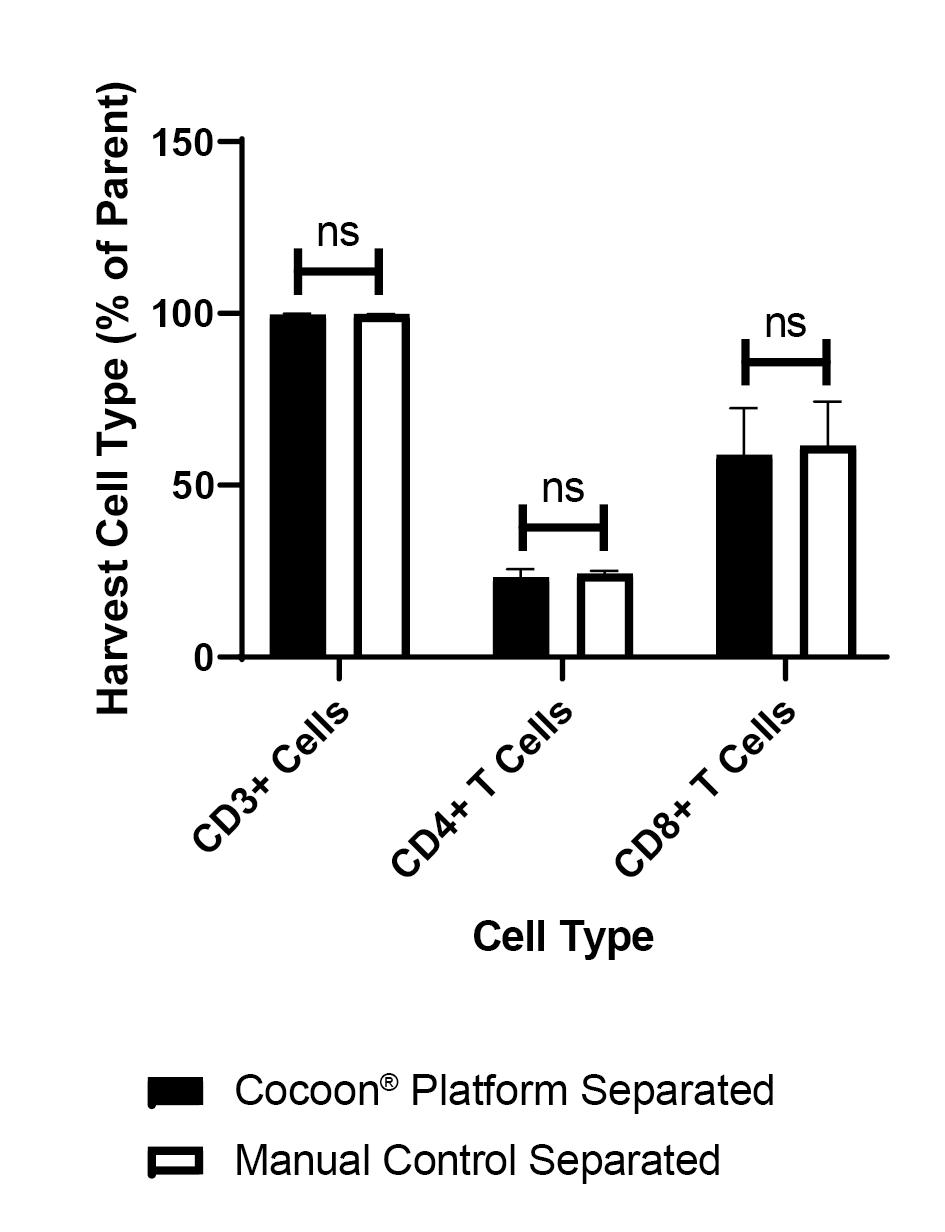
There is no significant difference between the new Cocoon® Platform and the manual control (Figure 8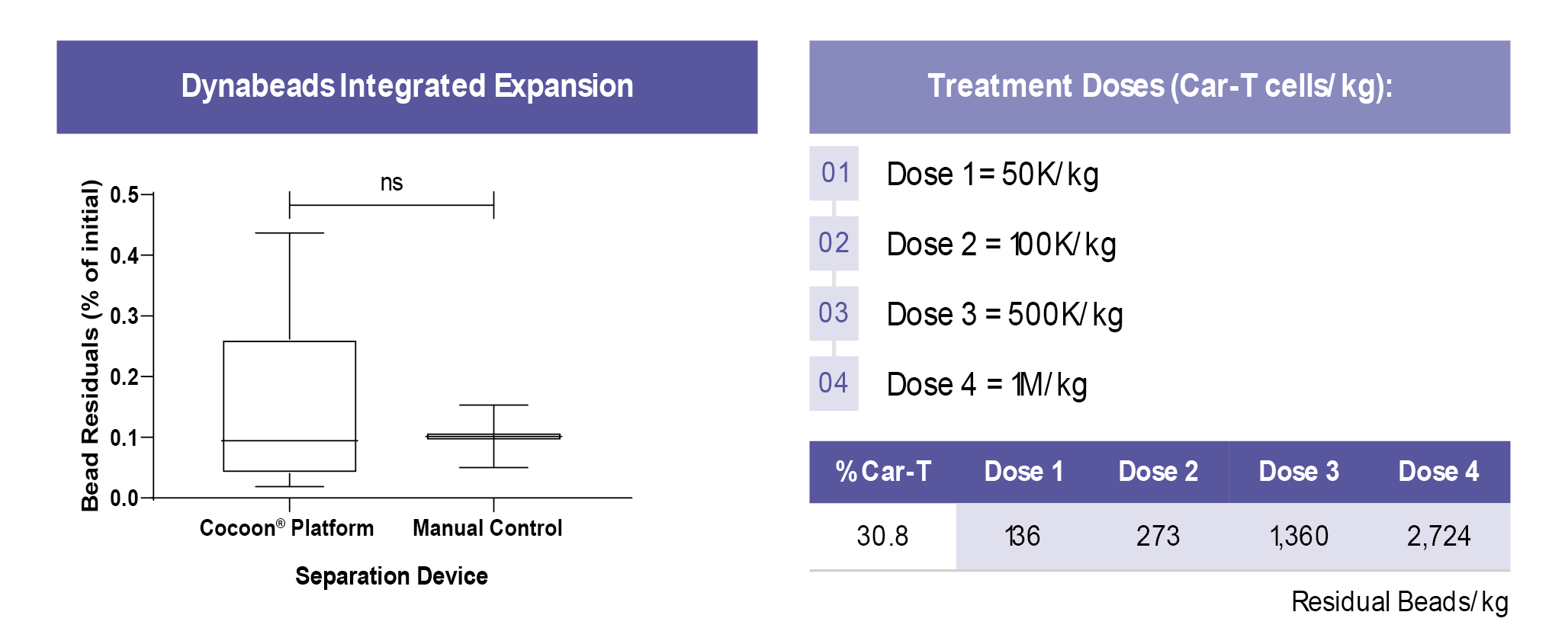
From a treatment perspective, even at the highest dose, the Cocoon® Platform can reach 35 times lower bead residuals than the currently used published standard of 96,000 beads/kg [7]White RD, Glosson JA, Gordon DE et al. Intravenous Safety Study in Rats Given Paramagnetic, Polystyrene Beads with Covalently Bound Sheep Anti-Mouse Immunoglobulin G (IgG). Int. J. Toxicol. 14(4): 251–65..
Key Insights
The Cocoon® Platform will allow for full automation of CAR T production for clinical scale at the Malaghan Institute in a flexible, closed manufacturing system. The platform’s new magnetic separation feature will allow for an efficient, automated bead removal step. Together, these will provide a fully scalable method of producing consistent cellular starting material for autologous cell therapies.
References
1. Neelapu SS, Locke FL, Bartlett NL, et al. Comparison of 2-year outcomes with CAR T cells (ZUMA-1) vs salvage chemotherapy in refractory large B-cell lymphoma. Blood Adv. 2021 Oct 26;5, 4149–4155. Crossref
2. Frederick L. Locke, David B et al. Primary Analysis of ZUMA-7: A Phase 3 Randomized Trial of Axicabtagene Ciloleucel (Axi-Cel) Versus Standard-of-Care Therapy in Patients with Relapsed/Refractory Large B-Cell Lymphoma. Blood 2021 138 (Supplement 1), 2.
3. Lai Y, Weng J, Wei X et al. Toll-like receptor 2 costimulation potentiates the antitumor efficacy of CAR T Cells. Leukemia 2018;32, 801-808. Crossref
4. Weng J, Lai P, Qin L et al. A novel generation 1928zT2 CAR T cells induce remission in extramedullary relapse of acute lymphoblastic leukemia. J. Hematol. Oncol. 2018, 20;11,25. Crossref
5. Weinkove R, George P, Ruka M, Haira TH, Giunti G. Chimeric antigen receptor T-cells in New Zealand: challenges and opportunities. N. Z. Med. J. 2021 Sep 17;134, 96–108. Crossref
6. George P, Dasyam N, Giunti G et al. Third-generation anti-CD19 chimeric antigen receptor T-cells incorporating a TLR2 domain for relapsed or refractory B-cell lymphoma: a phase I clinical trial protocol (ENABLE). BMJ Open. 2020;10,e034629. Crossref
7. White RD, Glosson JA, Gordon DE et al. Intravenous Safety Study in Rats Given Paramagnetic, Polystyrene Beads with Covalently Bound Sheep Anti-Mouse Immunoglobulin G (IgG). Int. J. Toxicol. 14(4): 251–65. Crossref
Ask the experts
 |  |
David McCall, Editor, BioInsights, speaks to (from left to right)
Rachel Perret, Team Leader, CAR T-cell Research Program, Malaghan Institute of Medical Research, and Kenneth Olsen, Senior R&D Scientist, Personalized Medicine Business Unit, Lonza.
Q What advantages did the team see in moving to fully automated systems?
RP: The main preliminary advantage we have seen is an increase in our production capacity, so that we can treat more patients faster. The two main reasons for this are that we can produce multiple CAR T cell products at the same time in our existing facility space, and that we can use our existing staff to be that capacity.
Q Can you expand on your experience with process translation into the Cocoon® Platform and the comparability study?
RP: We are still in the R&D phase at the moment. We are able to obtain pretty comparable results in the Cocoon® Platform with both healthy donor cells and patient cells, although patient cells are more difficult to work with.
We were able to work with the Lonza team to optimize the process as we went along and have found that we can achieve good CAR T cell expansion and good CAR expression in the Cocoon® Platform. The Cocoon® Platform is the logical next step for us to expand our CAR T cell production options at the Malaghan.
Q Does Malaghan have plans to develop additional therapies? Would process development (PD) be done in the Cocoon® Platform, or in another vessel?
RP: We hope to roll out further CAR T cell trials in the future. We are working on different strategies such as dual antigen targeting CAR T cells, and through our collaborations we have options to move into the solid tumor field in the future.
We would do all our early PD manually as it allows for more flexibility at the very small scale in early stages. Then we would transition to the Cocoon® Platform once we have the preliminary process in place.
Q Does Malaghan have plans to manufacture in Grade C or unclassified space at any point?
RP: Not currently. Our regulators require us to work in a Grade A tissue culture hood and a Grade B background for our open cell culture work. As parts of our process are still going to be open (leukapheresis processing and cell harvest), at present we need to remain in a Grade B background.
Q How many runs have you performed with the magnetic separation feature?
KO: We have done lots of partial runs in order to optimize the magnetic separation process. We are building on top of a process that already exists, and we know works quite well.
We have done 13 full runs from beginning to end with the magnetic separation feature, with several more in process currently.
Q How many types of beads have you tested on the Cocoon® Platform?
KO: Currently, most of our efforts on this project have been using Dynabeads. We have begun testing on several other different beads that are on the market, including some nano-scale beads.
Q Is the Cocoon® Platform with magnetic separation ready for sale?
KO: Currently, it is available for presale. If you contact us, we can begin that process.
We expect actual shipments of the Cocoon® Platform with the magnetic separation to be available in Q1 of next year.
Q Will Lonza develop bead solutions for dissolvable nanobeads?
KO: Currently, our strategy is to support a wide range of commercially available bead types, as we want our platform to be as flexible as possible. We do not have any specific plans for supporting dissolvable nanobeads. If this is something that is required for a particular process, we encourage scientists to reach out to us see if we can make this a solution.
Q How do you select T cells after obtaining PBMCs?
RP: We do a buffy coat (Lymphoprep™) separation, so PBMCs are obtained by density gradient. We then isolate the required T cells by magnetic positive selection using CD3/CD28 Dynabeads. This step is performed within the Cocoon® Platform during the automated process.
Q Why did you choose Cocoon® Platform?
RP: We looked at all the different options on the market; there are some other great technologies. The Cocoon® Platform had the advantage of being a small, single, and closed unit which fits well with our existing capacity and plans to scale-up. Also, it is compatible with the type of beads we were using for cell selection and activation.
Q What were the tweaks to enhance transduction efficiency?
RP: The Cocoon® Platform protocol was adapted from our fully optimized manual process, so it ran a lot more smoothly than the initial manual attempts, because we were far more advanced in our process at this stage. However, we had to do many tweaks, including to cell concentration, to the processing of cells, the way the lentivirus is added to the cells, as well as some of those little tweaks you get a feel for when working with your hands in the lab.
I cannot comment too much on the actual modifications that were made in the Cocoon® Platform because they were done by the developers in Canada.
Q Why do frozen cells expand better than fresh cells?
RP: We are not 100% sure about the reason behind this. We did not expect it, especially based on what happens with healthy donor cells.
Lymphoma patients have a very different leukocyte and neutrophil make-up to a healthy person, both due to the cancer and the pre-treatments they go through. It is most likely that an inhibitory or suppressive population is being lost during the freeze-thaw process, like granulocytes or monocytes, so we are getting a cleaner lymphocyte preparation.
Q Are the residual beads after removal still functional, or are they decomposed so that just non-functional magnetic particles are left?
RP: The beads are antibody-coated, which are not long-term stable conjugations, so the antibodies will detach from the beads and the beads detach from the cells. We can see under a microscope that the beads do not remain connected to the T cells. They are magnetic particles that we do not want to transfer to the patients in large numbers.
Q Why do you need to remove the beads?
KO: Our data shows that expansion increases following bead removal whether at day 3 or day 7. The mechanism for this is an ongoing investigation. The best cell yield seems to be when removing beads either day 3 or 4 but can be delayed if it’s better for your specific process or needs.
Q Can the Cocoon® Platform perform a de-beading step for the Dynabeads process?
KO: As Rachel mentioned, the antibodies are conjugated to the beads, so unfortunately there is no way to remove the beads from the cells at the beginning of the process. We need to wait until the beads naturally detach from the cells. Once the beads are removed from the system, we can proceed with the rest of the run without having beads inside the sample.
Q How long does the automated cell separation process take?
KO: The magnetic separation process takes approximately 12 minutes. We pass our sample through the magnetic separation line multiple times to increase the yield. The 12-minute run time is the total time it takes for the entire sample to pass through that magnetic separation line and collect the beads that have been pulled out, repeated multiple times.
Q Does the Cocoon® Platform do the bead mixing or does the user pre-mix cells?
KO: Currently, the process does involve mixing the beads with the cells outside of the Cocoon® Platform, then loading them into the cassette where the magnetic separation occurs. We are currently developing processes to try to automate this entire process.
Q Is the Cocoon® Platform process including magnetic separation available for other cell types such as NK or DC?
KO: Currently, the process is not optimized for those types of cells. The Cocoon® Platform itself is capable of working with many different cell types. If there is a specific need for a different cell type, our team can figure out if those specific cell types and processes have already been looked at or it seems like a viable solution.
Q Is the Cocoon® Platform an open development platform?
KO: While the end users will be capable of developing the processes, we have teams that can work with scientists to ensure that various steps are optimized and that the process is going to be as successful as possible.
Q Is the automation package 21CFR part 11 compliant?
KO: Yes, it is. That is one of the main features that we have made sure to have in the automated process, so that it can be used where needed in GMP spaces.
Q What is the multiplicity of infection (MOI) used?
RP: I believe that is an MOI of 4.
Q Your data show 99% bead removal. Can you achieve that after day 3?
KO: The 99%, or greater than 99%, data presented is bead removal at the very end of the process, just before final harvest on the last day of the run. We do an additional bead removal step, with which we can achieve the 99%.
Q What is the maximum input of cell number, and at what bead ratio?
KO: Currently the highest number of cells that we have used in the system is 450 million total CD3+ cells, at a bead ratio of 2:1. Our system is quite capable of handling roughly around 1 billion cells.
Q What are the minimum volumes on the Cocoon® Platform?
KO: During the beginning steps for introduction of cells into the cassette, the minimum number of cells that we recommend is 30 million cells, seeded, typically in 30ml of solution.
RP: Our process manual process has a starting number on the lower end of the scale. So, in the Cocoon® Platform we also start with the low number of cells at a concentration of about 1 million cells/ml. Due to media recirculation and continuous monitoring of oxygen content and pH levels in the Cocoon® Platform, we do not have to constantly count and re-seed cells at particular concentrations throughout the process. They can expand well with a constant flow of media circulating throughout the proliferation chamber.
Q Can you comment on the negative versus positive selection processes on the Cocoon® Platform?
KO: We have done a few preliminary studies looking at the differences between negative and positive selection – they both show good results. It really depends on your process needs. The negative selection can be more costly, but if a process requires that the targeted cells are not bound to magnetic beads then that might be the only solution. Our system is capable of managing that as well.
Q How many cells are you able to harvest at the end of your Cocoon® Platform?
RP: It depends on the patient starting material. We have had final products ranging from anything between 4×108 and 2×109 cells at the end of the process.
Q Is there a specific cassette for immunoselection?
KO: We have a specific cassette for magnetic separation. It is not available on the previous cassettes that we have been using. However, the new cassette that we are developing in connection with this magnetic separation capability will become the standard cassette moving forward. Whether or not the feature is needed, it will be available.
Q Are the magnets in the Cocoon® Platform developed to also work with smaller magnetic beads?
KO: Currently, most of our work has been done on the larger Dynabeads. However, we are beginning some investigative work on the smaller range. We are working on optimizing steps so that we can support both types of beads within the same system.
Q Could the Cocoon® Platform be compatible with transfection instead of transduction?
KO: I have not been involved in any of those studies currently, although we are looking into certain technologies that could be integrated with the Cocoon® Platform. We are going to expand the capabilities in the future, for more than just transduction.
Biographies
Rachel Perret, Team Leader, CAR T-cell Research Program, Malaghan Institute of Medical Research
Dr Rachel Perret is a Senior Cancer Research Fellow and Team Leader of the CAR T-cell Research Programme at New Zealand’s Malaghan Institute of Medical Research. She studied Microbiology at the University of Otago, NZ, before undertaking her PhD in Immunology with Professor Franca Ronchese at the Malaghan Institute. Rachel spent several years overseas, first at the Lausanne Branch of the Ludwig Institute for Cancer Research, and then at the Fred Hutchinson Cancer Research Center, and returned to the Malaghan Institute in January 2020. Her research focus lies in investigating chimeric antigen receptor (CAR) T-cell signalling and function, in order to design even better, safer, and more broadly applicable CAR T-cell therapies for cancer.
Kenneth Olsen, Senior R&D Scientist, Personalized Medicine Business Unit, Lonza
Dr Kenneth Olsen received his BSc in Biomedical Sciences, and his Masters in Biology and Vision Science from the University of Waterloo. He received his PhD from the Institute of Medical Sciences at the University of Toronto where he developed a novel device for generating biomechanical compression as a cellular model for glaucoma. After a postdoctoral research associate position at the University of Utah, and a Postdoctoral Fellow at the Canadian Centre for Alternative to Animal Methods at the University of Windsor, Dr Olsen joined the Lonza Personalized Medicine R&D team as a Senior Scientist in July 2021.
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: The authors have no conflicts of interest.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2022 Lonza AG. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: This article is a transcript of a webinar, which can be found here.
Webinar recorded: Nov 23 2021; Revised manuscript received: Feb 4 2022; Publication date: Feb 10 2022.


