Developing stem cell therapies: expansion strategies and lessons learned
Cell & Gene Therapy Insights 2022; 8(1), 113–126
10.18609/cgti.2022.024
The field of mesenchymal stromal cell therapy for critically ill patients has been evolving rapidly. In this article, Corning Field Applications Scientist Catherine Siler will present tools and process design strategies for achieving consistent and efficient expansion of therapeutic cells, including scale-up and scale-out options and closed system designs. Then, Ottawa Hospital Research Institute scientists Shirley Mei and Josée Champagne will put the technology in context by discussing how their team translated a mesenchymal stromal therapy manufacturing process from the preclinical laboratory to a GMP setting.
Expansion of mesenchymal stromal cells: technical challenges & solutions
Mesenchymal stem/stromal cells (MSCs) present an attractive target for cell therapies for several reasons, not least that they can be derived from multiple tissues, including adult adipose tissue and bone marrow, and newborn umbilical cord (Figure 1).
Challenges in MSC culture
As seen in (Figure1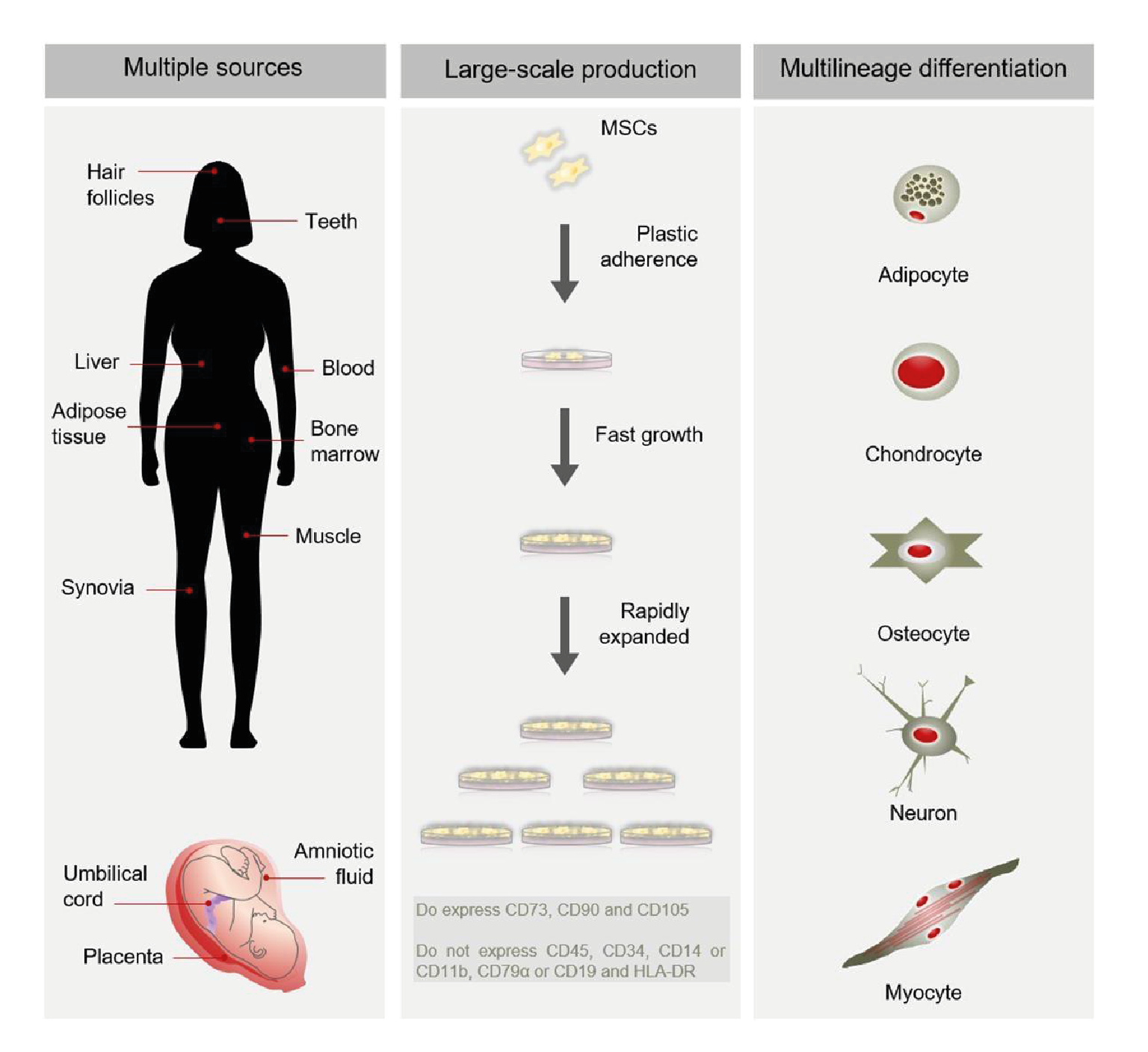
It is also important to consider the medium used. Traditionally, MSC culture medium would include either a serum component or, for those looking to stay xeno-free, human platelet lysate. Although both of those support robust culture, applications that are geared towards human therapeutics might ultimately steer away from any type of serum component. For those in a clinically oriented setting, there are several types of media available that are chemically defined, and therapeutic developers must test and choose the variety that best supports their culture goals.
Although MSCs grow well in adherent culture, it is important that they retain their phenotype and potency while in culture. For new processes, researchers may want to assess identity using some of the markers previously mentioned.
Perhaps the greatest challenge that MSCs present is the significant number of cells required to achieve therapeutic effects in vivo – up to 2 million cells per kilogram of body weight, adding up to hundreds of millions of cells for an adult patient. This requires a large number of vessels and could put pressure on the staffing or space in a facility. Hence, it’s critical to have a robust scale-up plan.
Manufacturing at scale
The first consideration in developing a seed train is what will keep the cells growing and functioning optimally. Some users may want to achieve a greater confluence before passaging, while others might want to prevent them from getting to a certain percentage confluence.
With that in mind, the starting material or cell bank is an important factor. MSCs can become senescent over time, and it is important to harvest their capabilities before their growth slows down, so population doublings are an important factor. It is also valuable to consider how many passages the cells will experience in the scale-up process because this has implications for both cell health and process efficiency.
Whatever the next step in vessel size or type, it is important to consider the consistency of the culture environment, including the ratio of media to surface area and the treatment of the surface the cells are growing on. Because the chemical structure of an untreated polystyrene is oily and very hydrophobic, many Corning® vessels feature the CellBIND® surface. The CellBIND treatment makes polystyrene more hydrophilic by the addition of oxygen-containing groups like hydroxyls, carboxyls, and carbonyls (Figure2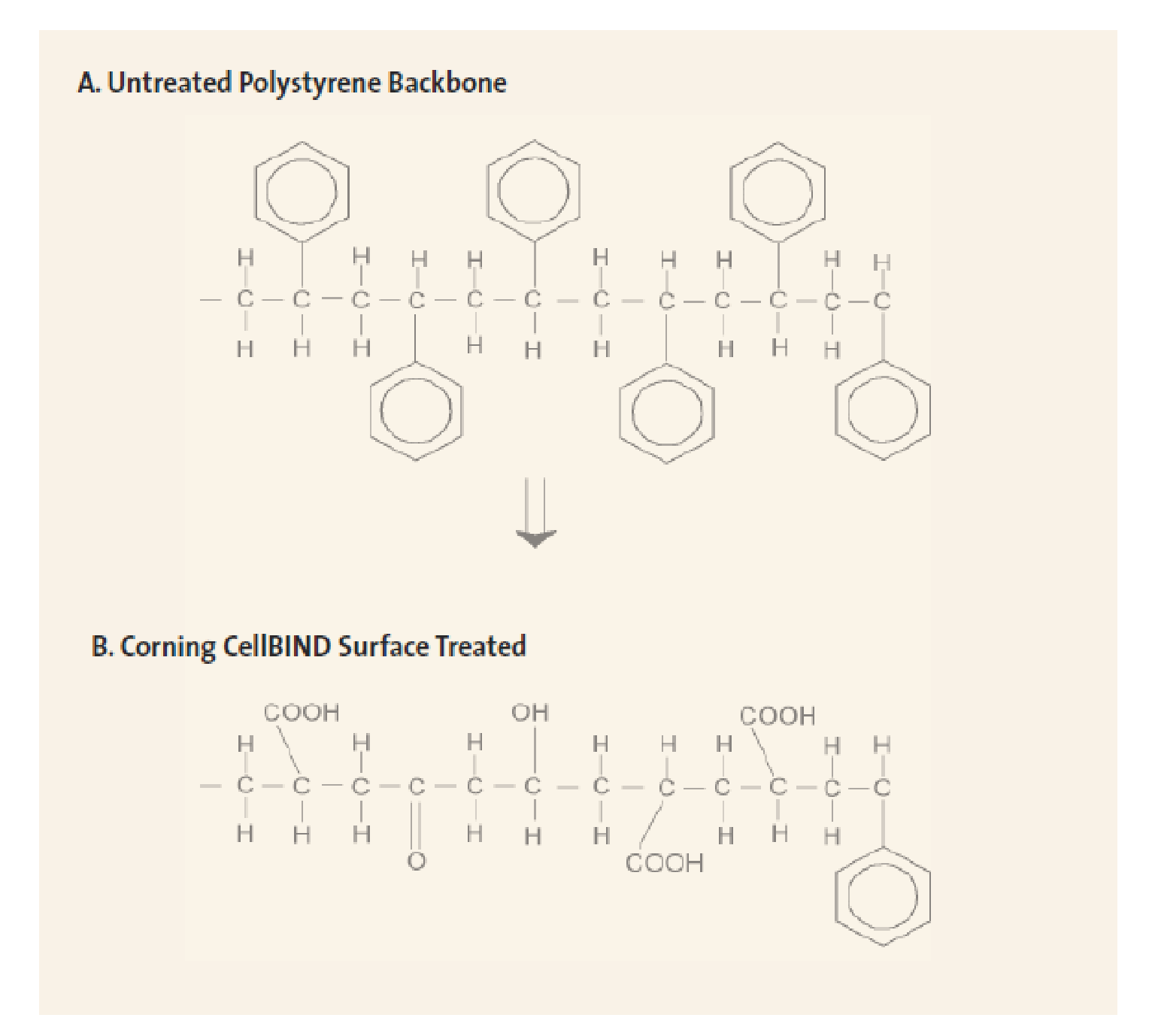
Scale-up or scale-out?
A final consideration for the seed train is the space in which a given process will occur, and therefore whether to scale out or scale up. Scale-out models add vessels of a similar size or type, whereas scale-up models introduce a larger vessel, which may be of a different type (Figure3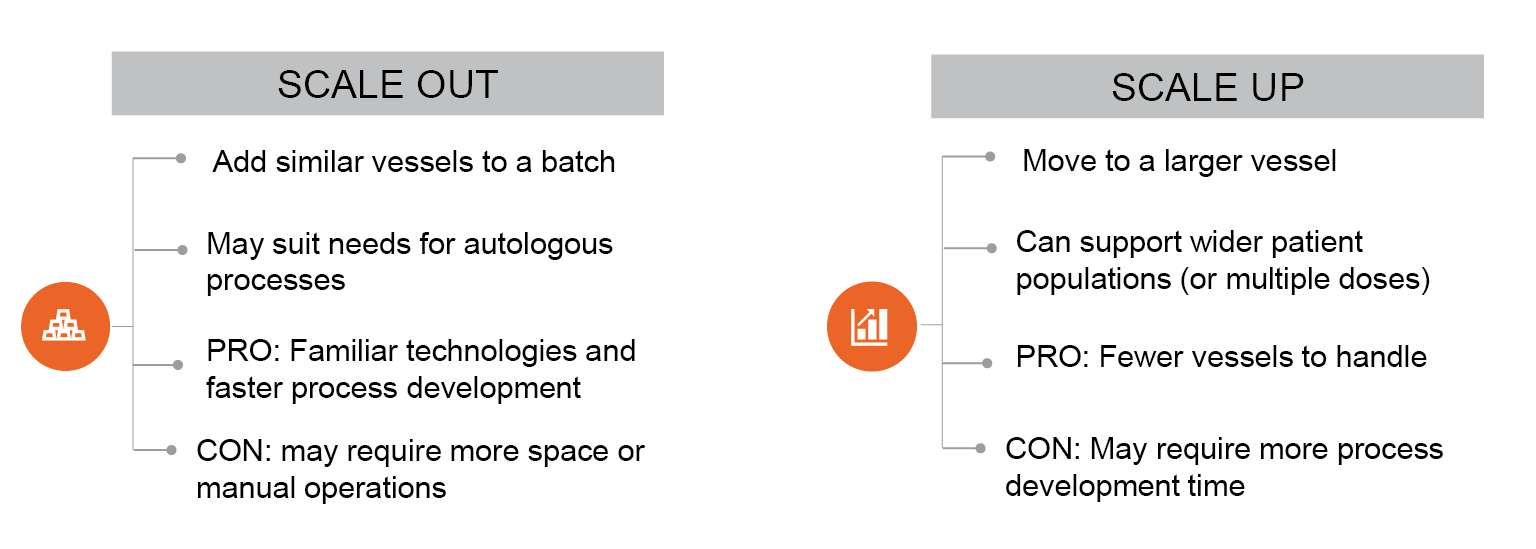
Technology for scale
Corning has a wide range of cell culture vessels for scale up or scale out, which can be seen in (Figure4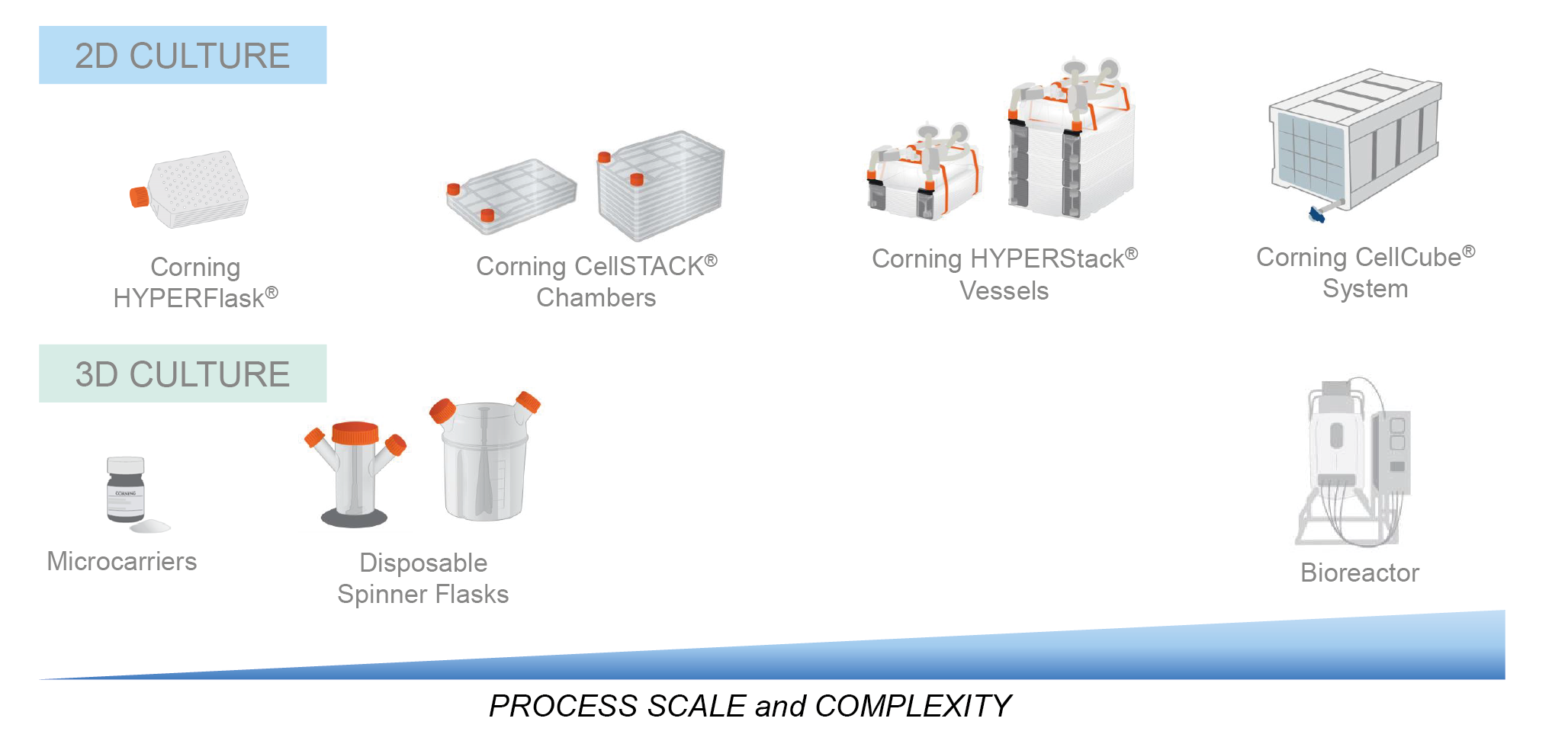
To demonstrate the advantages of the technology, HYPER vessels were employed in a seed train, which was designed with the goals of rapid expansion, a consistent cell culture surface, and minimal scale-up steps. Umbilical cord-derived MSCs were taken from thaw and cultured in a T-175 flask until they reached 90% confluence. After passaging, the cells were expanded into a HYPERFlask at a density of 3,000 cells per cm2 and after 5 days were passaged into a HYPERStack 36-layer vessel, with 18,000 cm2 of growth area.
The HYPERStack is a closed system vessel that allows users to transfer liquids more safely and can ultimately be used in GMP production. For clinical material, risk mitigation is critical, and closed systems play an important role in that process.
A surface area comparison can be seen in Table 1.
| Table 1. Surface area comparison for different vessel types | |||
| Vessel | Surface area | Equivalently sized vessel | Increased surface area |
| Corning® HYPERflask® | 1720 cm2 | T-175 | 10× |
| Corning® HYPERstack® -36 | 18,000 cm2 | Corning CellSTACK® -10 | 3× |
Over 60,000 cells per cm2 were recovered after five days of culture in the HYPERStack vessel. The average of three studies results in a total MSC yield of over 8.7 x 108 cells per HYPERStack-36, with consistent viability at 90%. Of note, the marker expression at the end of the process was consistent with the starting material and fits the criteria put forth by the International Society for Cellular Gene Therapy, with greater than 95% expression of CD105, 73, and 90, and no expression of typical hematopoietic markers.
Moving into larger scales of 10 billion cells or more, HYPERStack can be used in larger scale-out models such as those using manifolds and automated manipulators. At manufacturing scale, Corning offers bioreactor-related platforms such as microcarriers and the CellCube® system (Figure5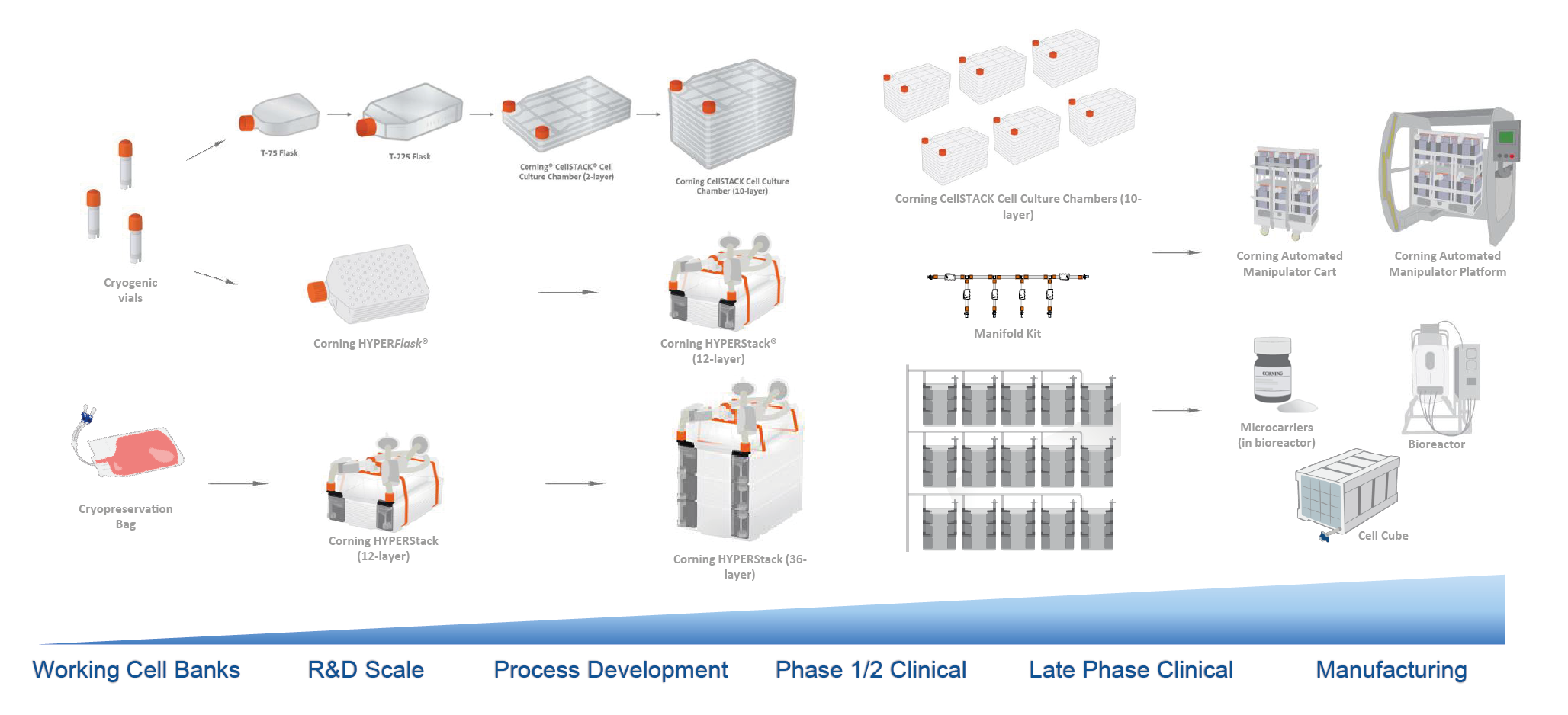
Clinical perspectives: developing a cell therapy for septic shock
Shirley Mei & Josée Champagne
Septic shock is a devastating illness. It is characterized by a highly dysregulated immune response, with cardiovascular collapse and organ failure. Despite early identification, aggressive resuscitation, and administration of antibiotics, patients still suffer a mortality rate of 20 – 40%. Those fortunate enough to survive face long-term morbidity associated with physical, cognitive, and emotional dysfunction.
From a healthcare system perspective, sepsis costs more than 4 billion dollars per year to treat and, despite decades of research, no targeted therapeutic agent for septic shock has improved clinical outcomes. Given the significant preclinical evidence, MSCs are believed to represent an exciting potential therapeutic option for this patient population.
In contrast to chronic diseases, acute and severe conditions such as septic shock require rapid intervention after disease is identified and this intervention must exert a therapeutic effect within hours of administration. Here, we discuss our progress in developing a MSC therapy for septic shock and how our manufacturing process evolved to meet the need for an off-the-shelf product.
Phase 1 trial: CISS1
After numerous promising preclinical studies by our own and other groups, the Regenerative Medicine Program at Ottawa Hospital Research Institute (OHRI) took on the challenge of developing a MSC product for testing in a septic shock clinical trial. Our Phase 1 trial, Cellular Immunotherapy for Septic Shock (CISS1), led by Dr Lauralyn McIntyre, was the first in the world to evaluate MSCs in septic shock patients.
The Phase 1, open-label, single-center dose-escalation trial used freshly cultured, allogeneic bone marrow-derived MSCs. The product was delivered as a single IV fusion to patients with septic shock, to examine the safety and tolerability of MSCs in this population setting.
Our secondary outcomes included the serial collection of biomarkers of inflammation over time and feasibility related to the operations and conduct of an MSC trial in this patient population.
Manufacturing
During the manufacturing development process, one of the biggest challenges initially for the team was on how to scale up from the small number of cells typically used in animal experiments to the large number of cells required to dose a patient.
We adopted a simple strategy, using a 10-layer Corning HYPERFlask. HYPERFlask vessels have around ten times the surface area of a similarly sized T175 flask and we found it can produce a proportional increase of cell yield. In addition, the similar size of a HYPERFlask to a standard T175 flask made the technology transfer process relatively simple when scaling up to the manufacturing facility.
(Figure6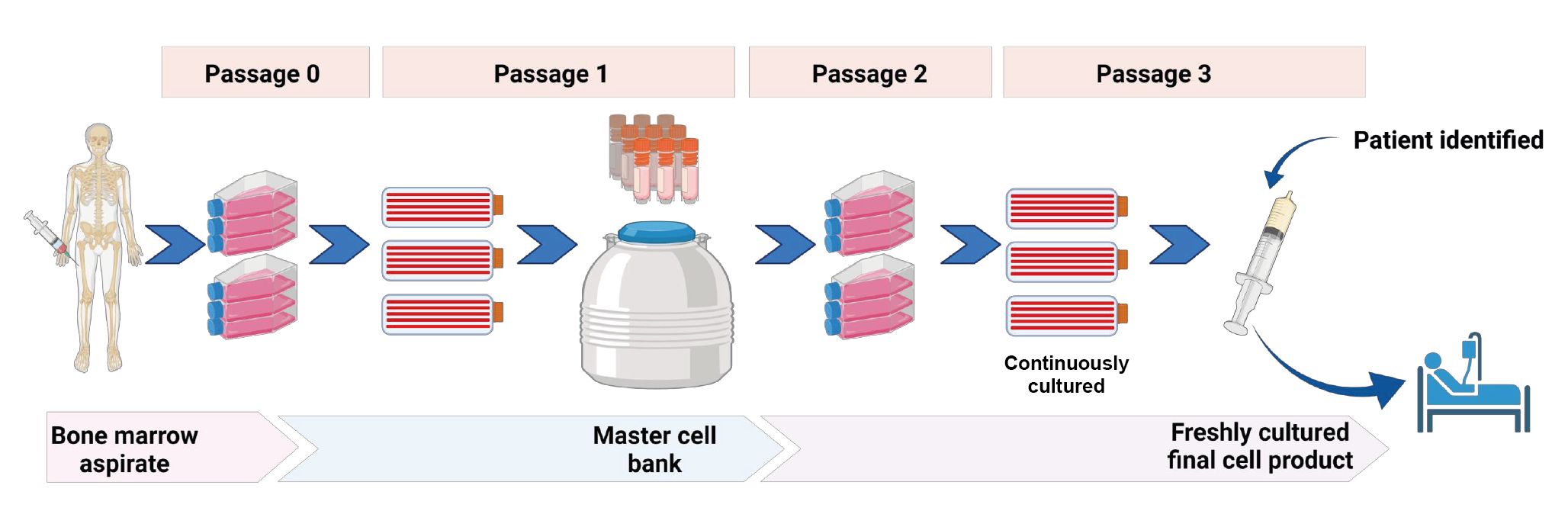
A master cell bank (MCB) was derived by seeding bone marrow aspirate from a healthy donor and the cells were further expanded to derive vials of MCB. The release specification for the MCB used in the CISS1 trial included but were not limited to cell counts, viability by hemocytometer count, surface marker expression, endotoxin, and sterility tests.
These MCB vials were subsequently used to seed a new set of HYPERFlask vessels weekly to allow a continuous supply of fresh cells. This strategy meant that the manufacturing facility need to harvest the cells on demand and deliver them to the ICU within a short time of an eligible patient being enrolled.
Given the very short time window to have the product ready to treat a patient, the release specification for the fresh MSC product used in the Phase 1 CISS trial included e a visual assessment for the appearance of MSC morphology, Trypan Blue count to confirm cell dose and concentration, and endotoxin level checks.
Trial design & results
Eligible septic patients were enrolled within 24 hours of first admission to the ICU. A control group of 21 participants who met the same eligibility criteria but did not receive MSCs was prospectively enrolled in advance of initiating the MSC interventional arm of the trial, to characterize the incidents of expected adverse events and serve as a comparator for the interventional group.
In the MSC interventional group, there were three separate MSC dose cohorts, with three participants per cohort, who received doses of 0.3, 1, or 3 x 106 cells per kg, to a maximum of 300 million MSCs.
Serial plasma samples were collected at various time points. Participants were monitored for MSC transfusion-associated events and serious unexpected adverse events for 1-year post-MSC transfusion. An independent data safety monitoring board (DSMB) reviewed the data following each cohort.
In conclusion, the Phase 1 trial determined that MSC doses of up to 3 million cells per kilogram appear safe, and that it was feasible to use MSCs in adult septic shock patients. We are now moving on to a much larger Phase 2, pan-Canadian, randomized, placebo-controlled trial [1]McIntyre LA, Stewart DJ, Mei SHJ et al. Cellular therapy for septic shock. A Phase I clinical trial. Am. J. Respir. Crit. Care MED. 2018; 197, 337–47..
Phase 2 trial: CISS2
The CISS1 trial showed that delivering freshly cultured cell products to septic patients, while possible, is difficult and can be costly. In particular, the use of fresh cell product could present challenges for hospitals without an established cell manufacturing facility within or nearby. The goal of our teams is to have the product ready to be administered at very short notice and it was clear that an off-the-shelf MSC product was needed to achieve that. Ahead of the Phase 2 trial, we started working on developing a process to cryopreserve the final cell product, so that it can be stored before administration.
Manufacturing
Further studies were required to confirm the comparability of fresh versus frozen cells, which will be used for the larger Phase 2 trial [2]Tan Y, Salkhordeh M, Wang JP et al. Thawed mesenchymal stem cell product shows comparable immunomodulatory potency to cultured cells in vitro and in polymicrobial septic animals. Sci. Rep. 2019; 9, 18078.Tan Y, Salkhordeh M, Wang JP et al. Thawed mesenchymal stem cell product shows comparable immunomodulatory potency to cultured cells in vitro and in polymicrobial septic animals. Sci. Rep. 2019; 9, 18078.. We compared cell recovery, viability, cell identity, and potency in vitro. Both exhibited similar surface marker profiles, viabilities, and in vitro potency. We then compared the potency of fresh and frozen products in an animal model of sepsis and found that both products were equally effective in recovering and even improving the phagocytic ability of the peritoneal lavage cells from septic mice (Figure7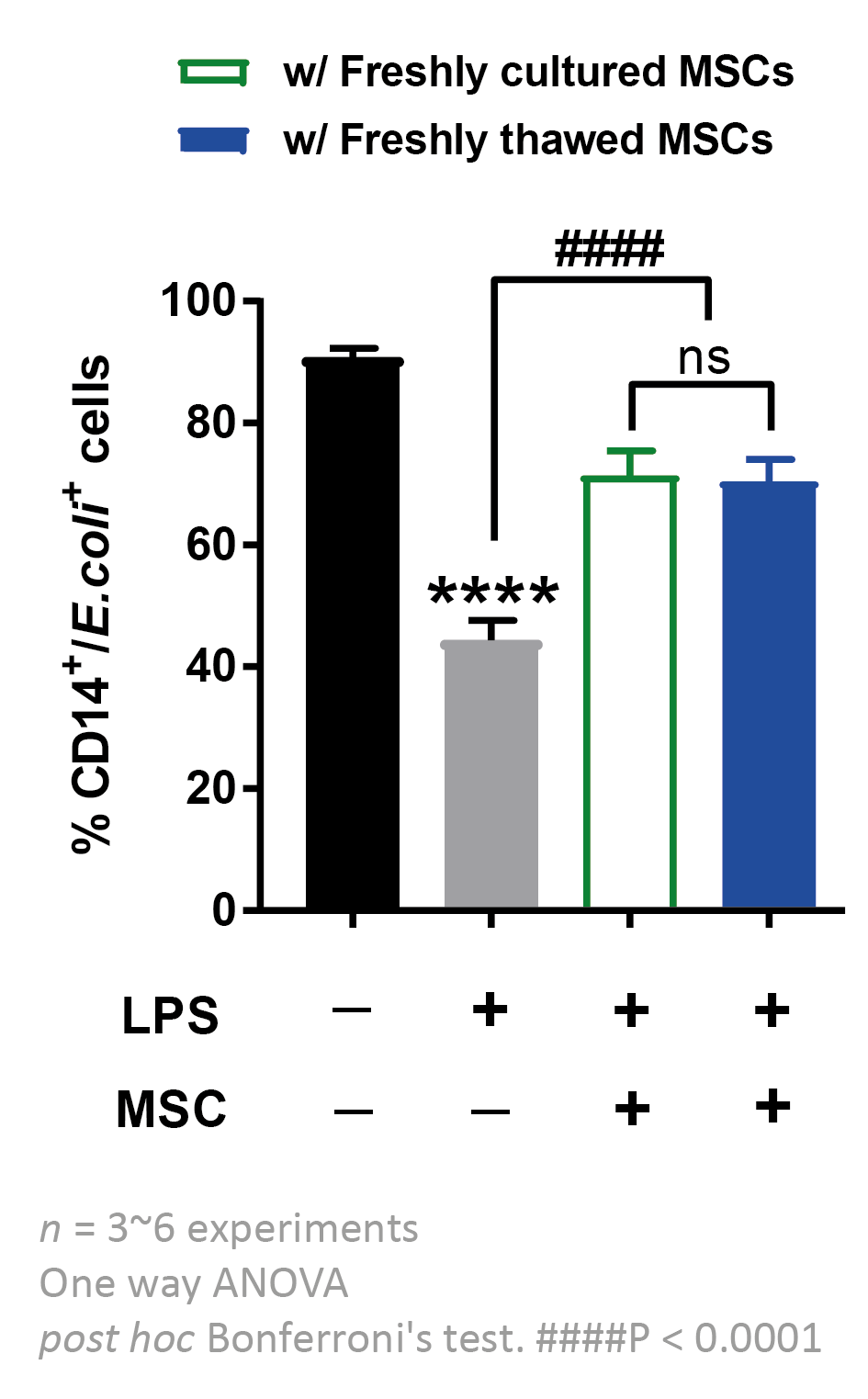
Taken together, these studies showed that the frozen product maintained good cell viability after cryopreservation and thawing, with similar functionality and potency to freshly harvested MSC.
Animal toxicology studies with the frozen MSC product also demonstrated comparability with fresh cells and showed no MSC-infusion-associated changes, no changes in kidney and liver function clinical chemistry parameters, no changes in body weight, and no changes in the histopathology of organ weight. Biodistribution studies found no trace of human genomic sequence at 60 days after the product infusion.
Trial design
The primary aim of the Phase 2 randomized, controlled trial (RCT) is to determine whether a single IV infusion of 300 million cryopreserved MSCs (the highest MSC dose from our CISS Phase 1 trial) reduces organ failures compared with placebo, in 122 patients across ten academic centers in Canada.
The frequency of adverse events and serious adverse events will be reported, and blood will be drawn for inflammation markers at baseline 12, 24, 72 hours, and 7 days post-infusion. The DSMB will be convened during the trial to review adverse events and/or clinical endpoints, and a priori adverse events.
Recently, the clinical trial protocol has been updated and reviewed by our executive committee, composed of multiple investigators across Canada, with the hope of submitting to Health Canada in January 2022, and we anticipate enrolling our first participant in Spring 2022.
Lessons learned from the translational process
When translating a promising lab research product from animal studies into human trials, think early and carefully about the product you want to use for your trial. Areas for consideration include:
- Logistics, including cold chain transportation, storage and transport.
- Type of patient population (acute vs chronic).
- Is the production process scalable beyond Phase 1 and possible to commercialize?
- Technology transfer: is your process transferable to a CDMO manufacturing facility?
- Cost of Goods: identify cost-saving opportunities during manufacturing process development, such as reducing media reagent, or developing a process with higher cell yield.
References
1. McIntyre LA, Stewart DJ, Mei SHJ et al. Cellular therapy for septic shock. A Phase I clinical trial. Am. J. Respir. Crit. Care MED. 2018; 197, 337–47. Crossref
2. Tan Y, Salkhordeh M, Wang JP et al. Thawed mesenchymal stem cell product shows comparable immunomodulatory potency to cultured cells in vitro and in polymicrobial septic animals. Sci. Rep. 2019; 9, 18078. Crossref
Ask the experts
 |  |  |
Charlotte Barker, Editor, BioInsights, speaks to (from left to right) Catherine Siler, Field Applications Scientist, Corning Life Sciences,
Josée Champagne, Senior Research Associate, Ottawa Hospital Research Institute, and Shirley Mei, Investigator, Ottawa Hospital Research Institute.
Q What do you see as the main advantages of using MSCs in cell therapy?
SM: The main advantage is that MSCs have been considered almost as a type of universal donor cell. Many cell therapies need the donor and recipient to be matched, but numerous clinical trials in various patient groups have not reported evidence of rejection with MSC cell therapy.
CS: The thing that fascinates me about MSCs is that many of the sources are fairly accessible. For example, adipose tissue is removed from people undergoing liposuction every day, and it is great to think that it can be used to create therapies when it would otherwise be discarded.
Then there are the various applications that it can be geared towards – areas like graft versus host disease, where there are few existing treatment options.
Q What are some of the safety considerations when designing an early-phase trial?
JC: We started by carrying out systematic reviews of preclinical and clinical studies to help identify (a) what type of a priori adverse events we could build into the trial protocol and (b) lessons learned from groups that have previously done similar work.
When designing the protocol, Dr McIntyre, along with the Canadian Critical Care Trials Group (CCCTG), were particularly mindful that adverse events in ICU-admitted septic shock patients occur daily; and it is very difficult to attribute causality. Instead, the protocol was designed to home in on (and define) what types of adverse events would be reportable. Furthermore, we also included stopping rules around the time of infusion that would, if required, pause recruitment until the data safety monitoring board (DSMB) had a chance to review. The protocol was also designed to allow for a DSMB review after each dose cohort before proceeding to the next dose. Prior to submitting the protocol for Health Canada review, we engaged the regulator early to ensure that our approach to safety was acceptable to them, especially for the first-in-human trial.
Q How do I translate my research workflow into a GMP-grade manufacturing process? What would be your key tips?
CS: One of the biggest transitions is the implementation of closed systems. Whether it’s something that you plan to continue doing in-house or outsource to a CDMO, implementing some type of closed system is always part of the conversation. Every process and every facility will probably have slightly different preferences in terms of what they want that closed system to look like. There’s really no one-size-fits-all, so we spend a lot of our time talking to customers to help them tailor a process to suit their goals.
Q What are the typical challenges and gaps that emerge when you’re having those detailed conversations?
CS: What works for you at the bench scale might not work for you once you get to manufacturing. You can implement a closed system at the bench scale, but those steps might not make sense at manufacturing scale, in terms of the facility or the employee resources, so it’s important to plan ahead.
In addition, we have a lot of discussions around making sure that everything works together. It’s not unusual that some components come from one vendor, some come from another, and all those pieces need to sit together.
Q What trends are you seeing in MSC research at the moment?
CS: One thing I’ve seen recently is that people are looking not only at MSC products, but MSC-derived products, for example, extracellular vesicles, which could potentially raise fewer immune concerns. I’ve definitely had a lot of conversations recently about acellular therapies.
Q How many passages of the master cell bank post-thaw can the process accommodate?
SM: It depends on the type of MSC (donor source, method of derivation) and how many passages you have processed before you bank it into the master cell bank. In our case, we don’t see much senescence in our MSCs until well beyond passage 7, so we keep our passages to 2 or 3, and the population doubling number relatively low. Ultimately, it’s up to individual researchers to understand your product.
Q How steep is the learning curve associated with a different scale-up technology?
CS: Moving from a smaller to a larger vessel in the same footprint, for example moving from T175s to CellSTACK®, is a quick way to get up to scale. Moving from an open to a closed system, like HYPERStack, that may be unfamiliar to users, will have a steeper learning curve. The steepest learning curve and the most process development time comes when you are implementing a bioreactor. For example, getting the cells to stick to a microcarrier, determining agitation conditions, and learning to work with whatever your chosen bioreactor platform is. The more advanced the technology, the more process development time is required but the benefits can also be significant.
Q How are MSCs extracted from donors safely, and how do you ensure the cells are healthy?
SM: As we’re doing allogeneic cell therapy, our cells are derived from bone marrow from healthy volunteer donors, not from patients themselves. We could give a whole seminar on how we pick our donors given the rigorous donor screening requirement – there is adventitious agent testing and a detailed review of the donor’s health history before we determine that they are suitable donors. We also carry out extensive testing of the master cell bank itself. The health of cells isolated can be determined not only by viability assessment but also through their potency and cell population doubling time.
Q Is there an active gas control on the HYPERStack or is it a passive diffusion through the vent filters on the top?
CS: The vent on the HYPERStack is there to facilitate liquid movement during emptying and filling. The tube leading to that vent filter is closed during culture. Otherwise, all of the gas exchange occurs passively through the sides of the vessel. No special gas setup is required – just a typical CO2 incubator.
Q What is the maximum amount of time that MSCs can be kept frozen and remain viable once thawed?
SM: Again, this is very product-specific, because cells may behave differently depending on tissue source and isolation method. You need to have a matrix of parameters to test your cells to know whether they are performing the same as fresh cells and confirm that you still feel comfortable using them.
For our cell product, we have stability data up to a year and for our master cell bank, we have 5+ years of stability data.
Q Which product performs better, the HYPERStack or bioreactors?
CS: Both will give you a lot of cells, so the decision really depends on your criteria for success, your critical quality attributes, and your process parameters.
If you determine that certain process parameters like the pH or concentration of specific metabolites must be very exact, a bioreactor gives you a method to control those parameters. But other factors, like the cost of goods, capabilities of your facility, and staffing, all have to be factored in. It really is very dependent on the user and what is important to them.
Biographies
Catherine Siler
Field Applications Scientist, Corning Life Sciences
Catherine Siler, PhD, is an accomplished Field Application Scientist for Corning Life Sciences, with a PhD in Biology from Johns Hopkins University. Siler enables scientists and researchers in the life science industry to overcome challenges with cell culture and scale-up for clinical manufacturing of advanced therapies, and utilizes her research and teaching experience to drive the adoption of an industry-leading global product portfolio of innovative single-use consumables for research, process development, and bioprocessing applications.
Josée Champagne
Senior Research Associate, Ottawa Hospital Research Institute
Josee Champagne has 20+ years of experience in both investigator-led and industry-sponsored clinical research. As the International and Regulatory Coordinator for the investigator-led, Phase 3 international Folic Acid Clinical Trial (FACT) she oversaw all aspects of the study, including participant recruitment, trial implementation, drug manufacturing, and importation, and closeout across 96 clinical sites in five different countries. Since 2016, she has been managing the Cellular Immunotherapy in Septic Shock (CISS) Program and has gained considerable expertise interpreting federal regulatory requirements for emerging biotech products, including cell and gene therapies.
Shirley Mei
Investigator, Ottawa Hospital Research Institute
Shirley Mei, PhD, MSc, has 18 years of experience in the nonclinical development and translation of MSC therapy into the clinic for critical illnesses. As a translational scientist, she has been working with ICU clinicians to conduct the worlds’ first-in-human clinical trial for septic shock patients with allogeneic MSC derived from bone marrow (NCT02421484), in addition to several ongoing cell-based gene therapy trials for critically ill or pulmonary patients. With a focus on bringing discoveries from the bench to the bedside, her current research includes studying how MSCs exert their immunomodulatory effect on immune cells using in vitro culture systems, preclinical disease models, and clinical samples; and developing novel molecular strategies to enhance the benefit of cellular therapeutics. Her lab also conducts essential translational research, including the development of GMP-compliant cellular therapeutic products and bringing products to clinics.
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: The authors have no conflicts of interest.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2022 Corning, Mei & Champagne. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: This article is a transcript of a webinar, which can be found here.
Webinar recorded: Dec 8 2021; Revised manuscript received: Feb 4 2022; Publication date: Feb 23 2022.


