The EuLV® System, an inducible stable producer cell line for lentiviral vector production
Cell & Gene Therapy Insights 2022; 8(2), 199 – 209
10.18609/cgti.2022.022
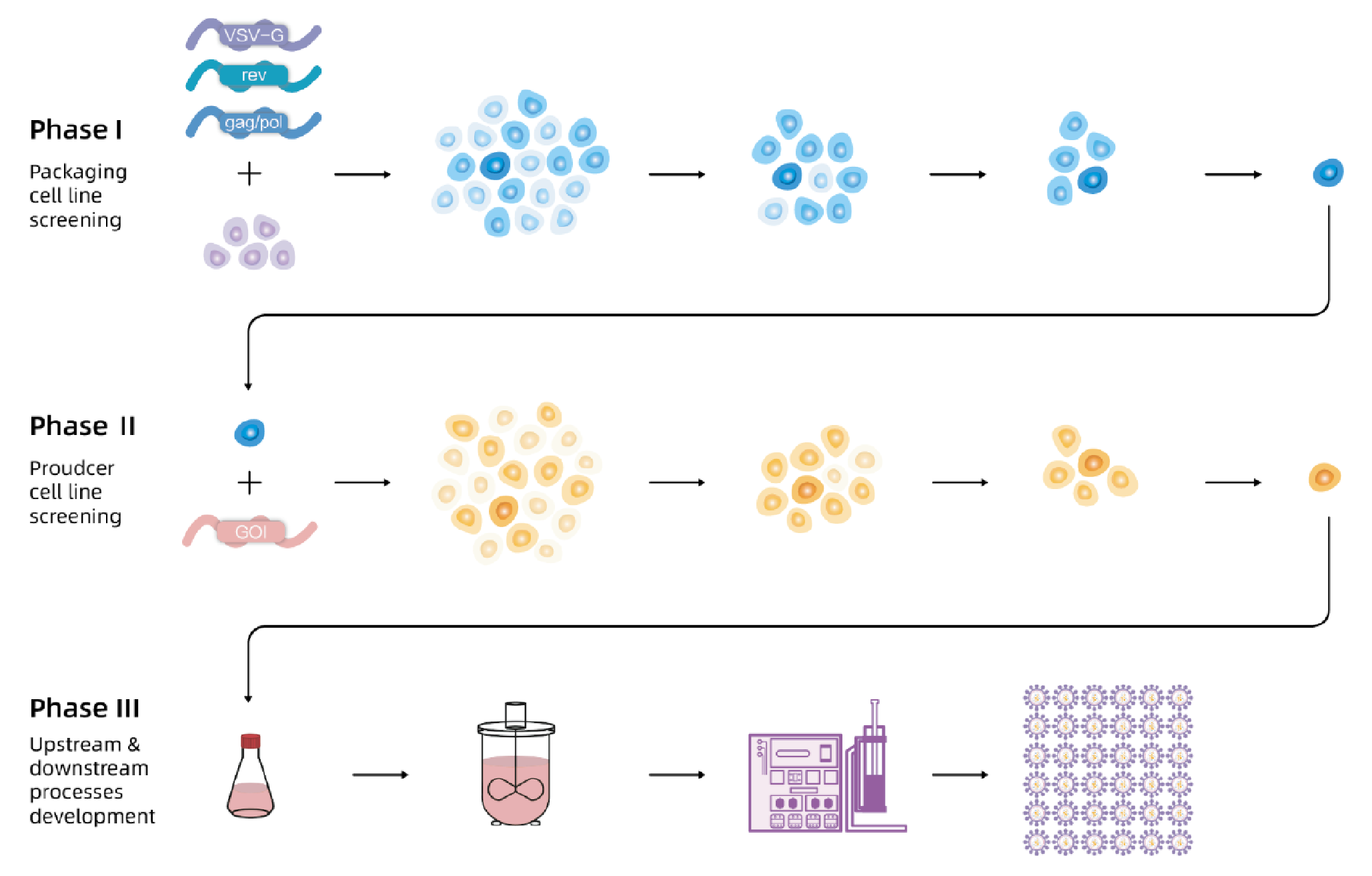
Lentiviral vectors (LVV) are widely used in gene and cell therapy. A stable cell line based production technology is crucial to the gene and cell therapy industry. This article introduces a stable producer cell line for LVV and its performance. In Phase 1, the packaging cell line, with lentiviral packaging genes gag/pol, rev and VSV-G is developed. In Phase 2, the producer cell line, with GOI stably inserted is developed. In Phase 3, all upstream and downstream processes based on stable producer cell line are developed.
Background
There are two methods for producing lentivirus, one of which is transient transfection, using different transfection reagents that can form complexes with DNA, allowing cells to take them up through endocytosis. This method is widely used clinically, but due to its low titre and difficulty in scaling up, it cannot meet the current industrial needs.
Another method of lentivirus production is using stable producer cell lines. Due to the low efficiency of stable gene insertion, the reported methods for constructing stable producer cell lines usually require introducing a large number of resistance genes for simultaneous or step-by-step screening. As a result of insufficient regulation and expression optimization of each lentiviral packaging gene, the final stable cell line may have high leaky expression during the construction of producer cell lines, resulting in unstable producer cell lines. These are unacceptable for pharmaceutical industry.
EuLV® system
The EuLV® system produces lentiviral vectors using a stable producer cell line that enables high-density cell culture and inducible lentiviral production in chemically defined media with low uninduced leakage of producer cells to manageable levels (Supplementary Data 1).
The flow chart of EuLV® system to construct the producer cell line is shown in Figure 1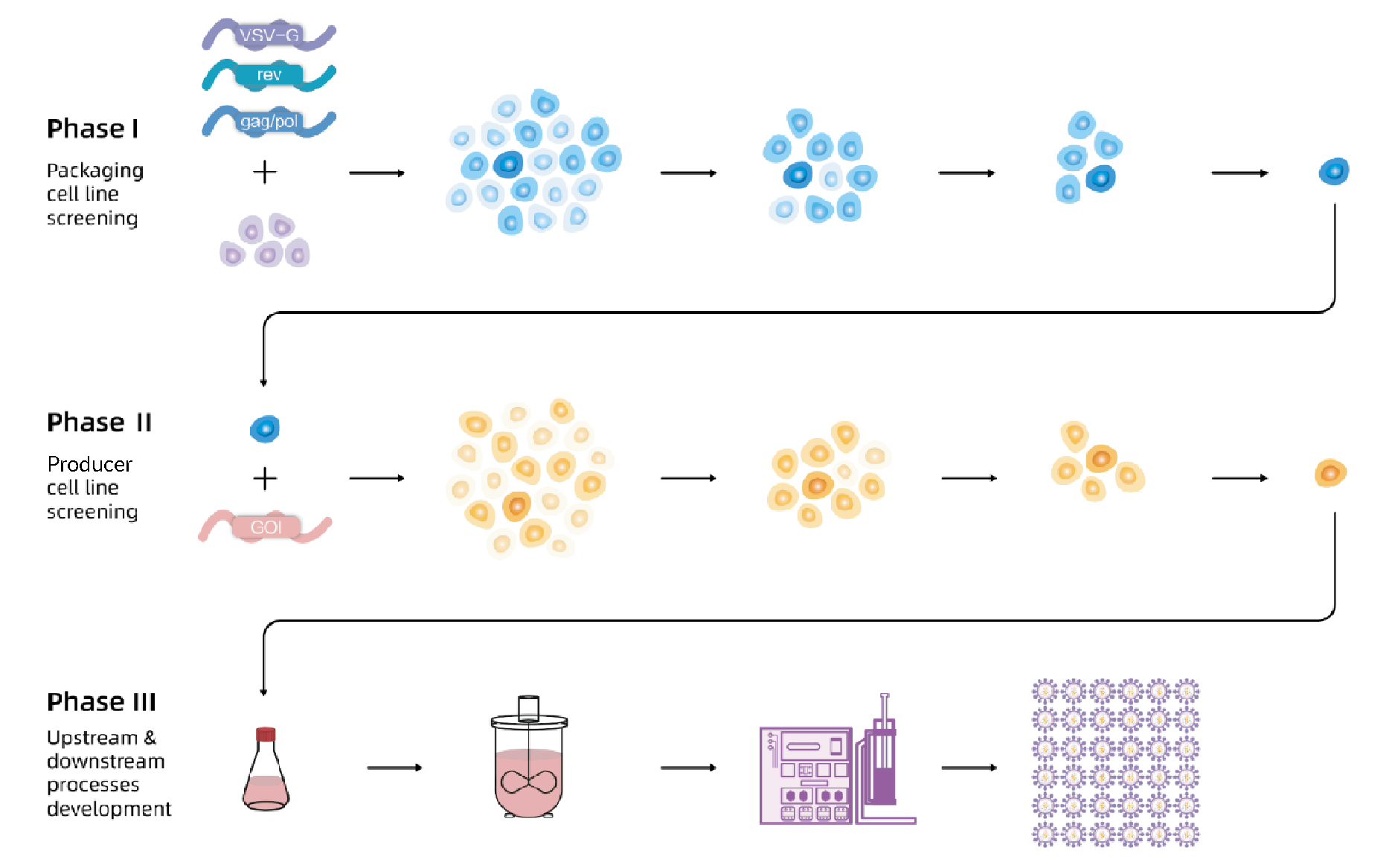 In Phase 1, the packaging cell line with lentiviral packaging genes gag/pol, rev and VSV-G is developed.In Phase 2, the producer cell line is developed with GOI stably inserted. In Phase 3, all upstream and downstream processes based on stable producer cell lines are developed.. First, VSV-G, gag/pol, and rev were integrated into 293T cells, and the optimal packaging cell line was obtained through monoclonal and titre screening; then, the viral genome transcription cassette carrying target nucleic acid fragment was integrated into the packaging cells, after monoclonal and titre screening, the optimal producer cell line was obtained. Finally, production and purification processes were developed based on producer cell lines to obtain high-titre and high-quality lentiviral vectors.
In Phase 1, the packaging cell line with lentiviral packaging genes gag/pol, rev and VSV-G is developed.In Phase 2, the producer cell line is developed with GOI stably inserted. In Phase 3, all upstream and downstream processes based on stable producer cell lines are developed.. First, VSV-G, gag/pol, and rev were integrated into 293T cells, and the optimal packaging cell line was obtained through monoclonal and titre screening; then, the viral genome transcription cassette carrying target nucleic acid fragment was integrated into the packaging cells, after monoclonal and titre screening, the optimal producer cell line was obtained. Finally, production and purification processes were developed based on producer cell lines to obtain high-titre and high-quality lentiviral vectors.
Generation of packing cell line & producer cell line
VSV-G, gag/pol, and rev were stably inserted into 293T cells to obtain packaging cell populations, which were screened for monoclonal cells using EuBioX (Supplementary Data 2), best 10 high yielding cells were chosen by transient transfection using hPGK-luciferase- IRES-EGFP plasmids (Figure 2 The hPGK-luciferase-IRES-EGFP (plasmid 19BF081) expression cassette is the Gene of Interest (GOI) , which is used for packaging cell line screening (single-plasmid transient transfection) and evaluation as well as producer cell line construction.Luciferase assay is used to quantify the transduction titer (TU (RLU) /mL) of the produced lentivirus.). Then hPGK-luciferase- IRES-EGFP was stably integrated into the chosen packing cell to obtain a population of producer cells, and the same procedure was performed to get high-yield producer cells. The virus titre during the screening process is shown in Figure 3
The hPGK-luciferase-IRES-EGFP (plasmid 19BF081) expression cassette is the Gene of Interest (GOI) , which is used for packaging cell line screening (single-plasmid transient transfection) and evaluation as well as producer cell line construction.Luciferase assay is used to quantify the transduction titer (TU (RLU) /mL) of the produced lentivirus.). Then hPGK-luciferase- IRES-EGFP was stably integrated into the chosen packing cell to obtain a population of producer cells, and the same procedure was performed to get high-yield producer cells. The virus titre during the screening process is shown in Figure 3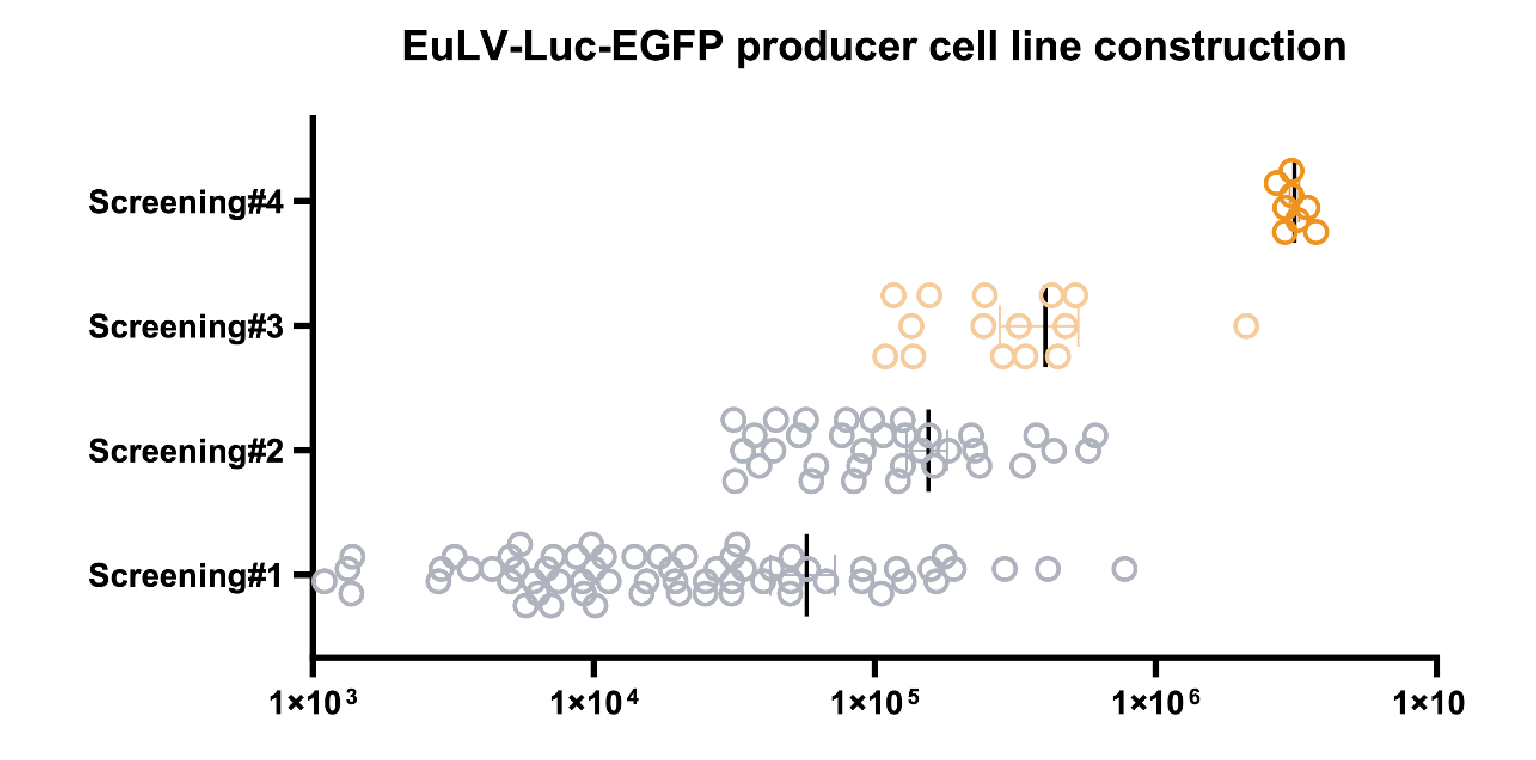 The viral titer from each clone in the process of producer cell line screening.The x-axis shows the transduction titer measured from luciferase assay, and four rounds of screening are performed. The average titer increases nearly 100 folds after the fourth screening step. One of the best producer clones is selected to develop the lentiviral production process..
The viral titer from each clone in the process of producer cell line screening.The x-axis shows the transduction titer measured from luciferase assay, and four rounds of screening are performed. The average titer increases nearly 100 folds after the fourth screening step. One of the best producer clones is selected to develop the lentiviral production process..
Cell culture
Freestyle 293 and other 6 commercially available CDM medium was tested for lentivirus production and CDM#2 was chosen (Supplementary Data 3). The producer cells are grown in suspension in shake flasks with an agitation of 170 rpm using 2.6cm orbital shakers, 8% CO2 at 37 °C. Cells were passaged to 0.5×106/mL when the cell density reached 4×106~6×106/mL.
Medium, feed & inducer
In many lentivirus production processes, the fresh medium needs to be replaced before induction or lentivirus production. These steps hinder the scale-up of the process. In EuLV®️system, media, inducers, and feeds were screened and optimized, and a feed batch method for lentiviral production was developed (Supplementary Data 4).
LV production in tube & flask
The producer cells were inoculated into 50mL tubes or shake flasks at a density of 0.5×106/mL, and cultured for 5 days until the cell density reached about 1.2×107/mL, then the inducer and feed were added to start the lentivirus production, feed was added again after 24 hours, and the supernatant was harvested after another 24 hours. The supernatant titre results in Figure 4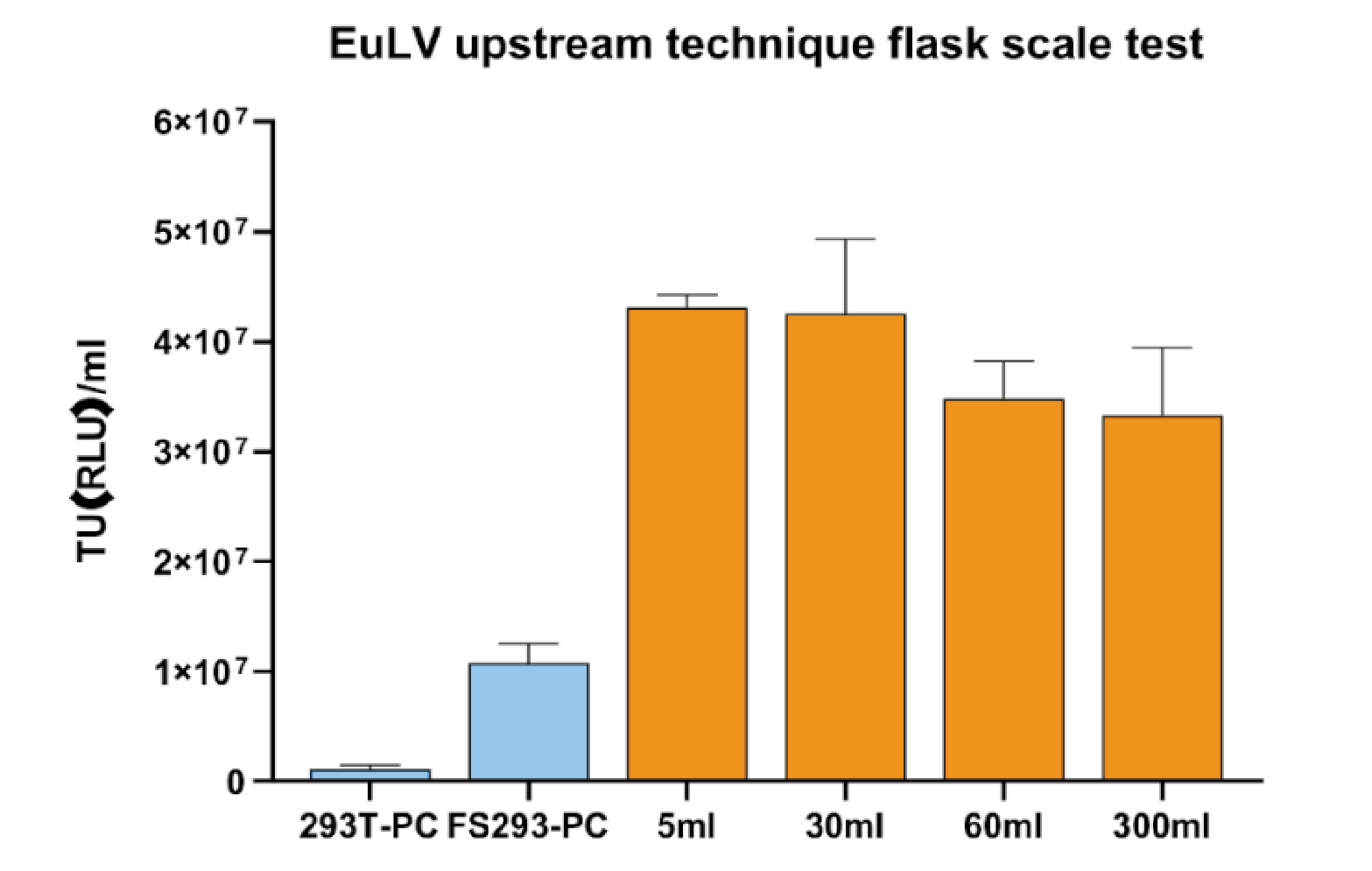 Viral titer produced in this process can increase 4 folds compared to the condition in FS293 medium (induced at 4 106 cell/mL with 100% medium re-placement) , and this process is stable when tested in the shaking flask scale..
Viral titer produced in this process can increase 4 folds compared to the condition in FS293 medium (induced at 4 106 cell/mL with 100% medium re-placement) , and this process is stable when tested in the shaking flask scale..
LV production in WAVE bioreactor
Producer cells were seeded into WAVE bioreactors at 0.5×106/mL in a total reaction volume of 1L. The reaction conditions were 37°C, 8% CO2, the stirring speed was 20 rpm, the angle was 10°, and the ventilation rate was 0.1 L/min. For the 1L reaction system, cells were directly inoculated into a 1L reaction system and induced to produce lentivirus when the induction density was reached (Figure 5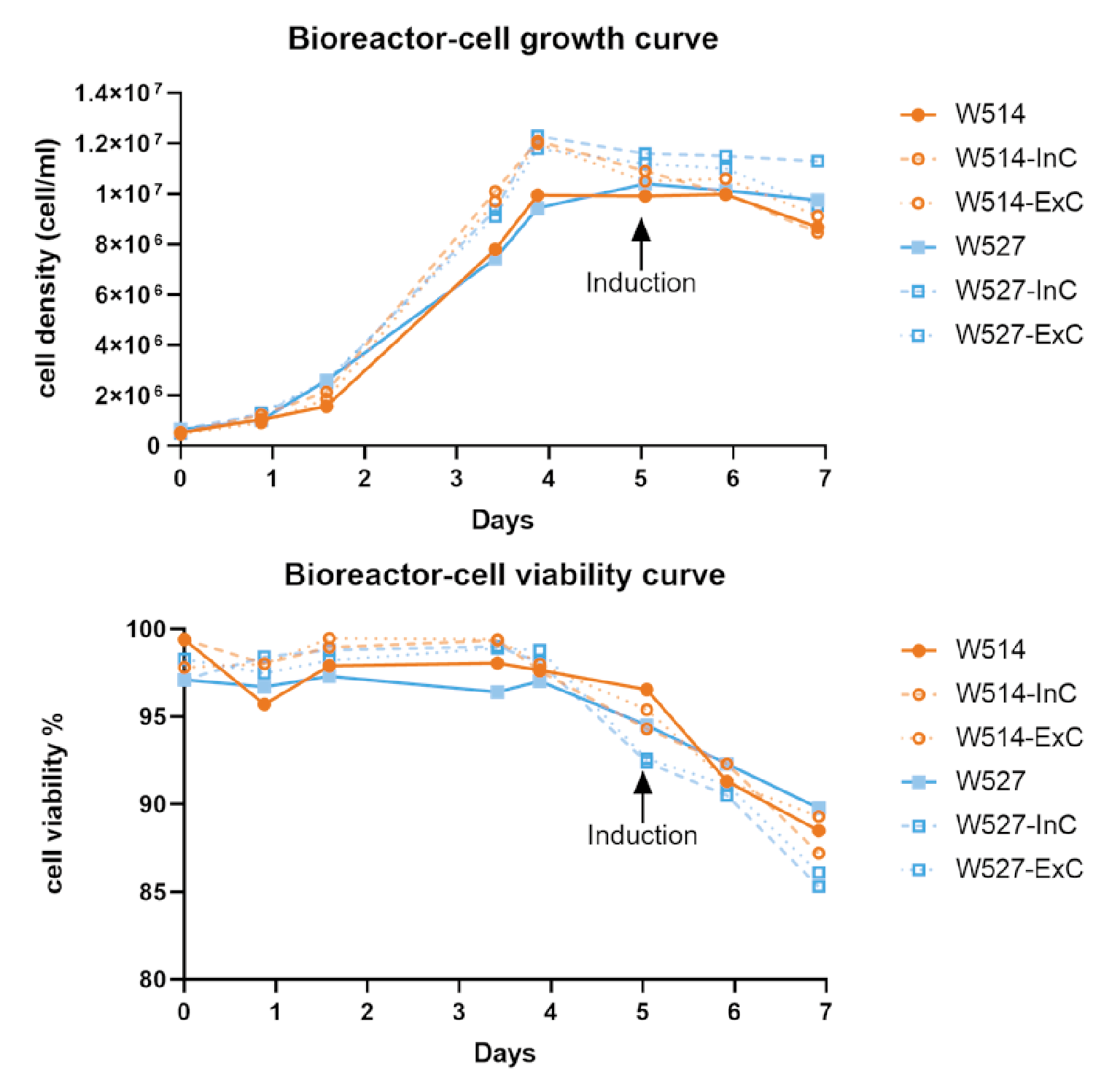 Results of two batches of 1L-scale lentiviral production using EuLV stable producer cell line in a WAVE20/50 bioreactor, and summarize the cell density and viability data during the 7-day culture and virus production period.W514 (orange) and W527 (blue) are independent batches. InC stands for internal control, a 20 mL sample collected after cells are filled into the culture bag; ExC stands for external control, a 20 mL sample prepared from the same batch of seed. Both InC and ExC follow the same procedure as the WAVE bioreactor in a CO2 shaker for culture and virus production. On day 0, cells are seeded at a density of 0.5 106 cell/mL and continue to expand until day 5, when the inducer is added. 48 hours later, on day 7, the virus in the culture medium is harvested and purified in the downstream process. The cell viability on day 7 remains above 85%, which is obviously beneficial to the downstream process.). For the 25L reaction system, cells were first inoculated into a 1L reaction system, cultured for 3 days, then expanded to a 5L system, cultured for 3 days, and finally expanded into a 25L system, as shown in Figure 6
Results of two batches of 1L-scale lentiviral production using EuLV stable producer cell line in a WAVE20/50 bioreactor, and summarize the cell density and viability data during the 7-day culture and virus production period.W514 (orange) and W527 (blue) are independent batches. InC stands for internal control, a 20 mL sample collected after cells are filled into the culture bag; ExC stands for external control, a 20 mL sample prepared from the same batch of seed. Both InC and ExC follow the same procedure as the WAVE bioreactor in a CO2 shaker for culture and virus production. On day 0, cells are seeded at a density of 0.5 106 cell/mL and continue to expand until day 5, when the inducer is added. 48 hours later, on day 7, the virus in the culture medium is harvested and purified in the downstream process. The cell viability on day 7 remains above 85%, which is obviously beneficial to the downstream process.). For the 25L reaction system, cells were first inoculated into a 1L reaction system, cultured for 3 days, then expanded to a 5L system, cultured for 3 days, and finally expanded into a 25L system, as shown in Figure 6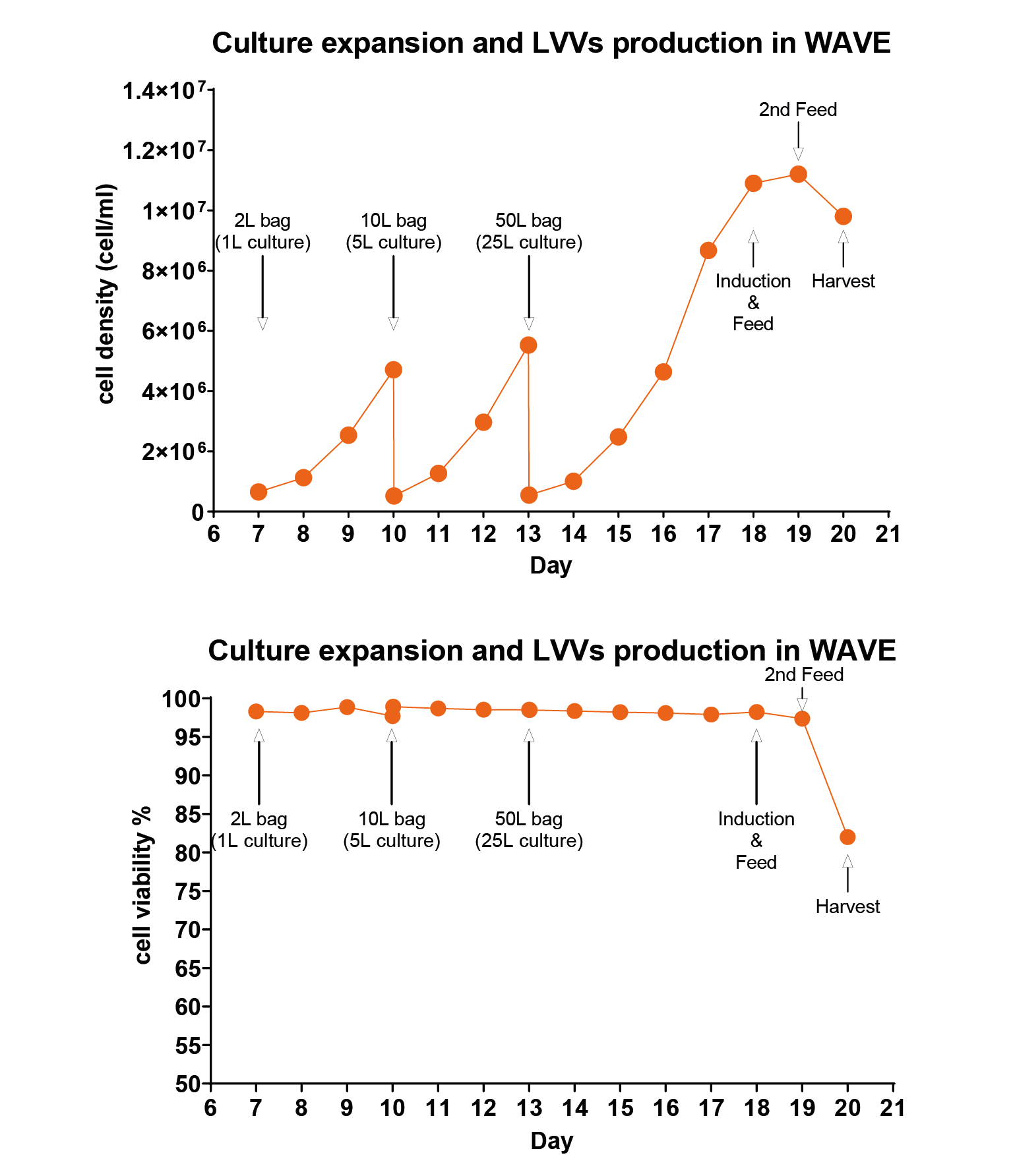 Cell density and viability data when the producer cells were amplified and induced for LVV production in the WAVE bioreactor.Calculated as day 0 from the recovery of cells, on days 10 and 13, when the cell density reached around 5 million per mL, the cells were transferred to the next size of wave bag, and on day 18, inducer was added for the virus production. Same as the 1L data, cell viability is controlled over 80% upon harvest. And for the virus titer, we detected 3.8 108 TU/mL in the culture medium, 9.5 1012 TU/mL before harvest, and 3.4 1012 after purification.. The cells were cultured in the final system for 5 days, and the cell density reached about 1×107/mL. The inducer and feed were added, and the culture was continued for 24 hours. The feed was added for the second time, and the supernatant was harvested after 24 hours.
Cell density and viability data when the producer cells were amplified and induced for LVV production in the WAVE bioreactor.Calculated as day 0 from the recovery of cells, on days 10 and 13, when the cell density reached around 5 million per mL, the cells were transferred to the next size of wave bag, and on day 18, inducer was added for the virus production. Same as the 1L data, cell viability is controlled over 80% upon harvest. And for the virus titer, we detected 3.8 108 TU/mL in the culture medium, 9.5 1012 TU/mL before harvest, and 3.4 1012 after purification.. The cells were cultured in the final system for 5 days, and the cell density reached about 1×107/mL. The inducer and feed were added, and the culture was continued for 24 hours. The feed was added for the second time, and the supernatant was harvested after 24 hours.
Purification
The purification steps are shown in Table 1. First, Clarification by centrifugation or depth filtration and microfiltration to remove impurities such as cell debris (step 1). Then, the purified lentivirus was obtained by ion-exchange chromatography (step 2), ultrafiltration concentration washing (step 3), gel filtration chromatography (step 4), and sterile filtration (step 5). See Supplementary Data 5 for the lentiviral purity profile.
| Table 1. Key data in the main purification steps from two 1L-batch of virus production. | ||||
| Process flow | Purity % | TU titer 1E8TU(RLU)/ml | Viral activity 1E6TU/ng p24 | Inducer residue |
| Medium | 1.55 ± 0.09 | 5.31 ± 1.73 | 1.92 ± 0.84 | N/A |
| Step 2 | 73.61 ± 4.95 | 3.26 ± 1.03 | 1.89 ± 0.89 | N/A |
| Step 3 | 97.86 ± 0.76 | 28.60 ± 5.94 | 2.21 ± 1.05 | N/A |
| Step 5 | 97.94 ± 1.07 | 11.31 ± 4.24 | 2.02 ± 1.03 | Not detected |
| The purity is measured by HPLC-SEC column. The transduction titer (TU titer) is measured by luciferase assay. The recovery rate based on physical titer (recovery-VP) is calculated based on the integration of peak area in the HPLC-SEC column analysis. The recovery rate based on transduction titer (recovery-TU) is calculated based on the luciferase assay. The virus activity is calculated by dividing the TU titer by the physical titer measured by ELSIA-based P24 protein quantitation method. The result is illustrated as transduction unit per ng of p24 protein. | ||||
ANTI-CD19 CAR-IE producer cell line
We also constructed the EuLV® antiCD19 CAR-IRES-EGFP producer cell line (Figure 7 The map of the GOI sequence has the typical anti CD19 CAR [1] sequence with additional IRES EGFP sequence.It is almost twice the length of the original anti CD19 CAR sequence.). Cell screening and process optimization are similar to hPGK-luciferase-IRES-EGFP. Results are shown in Figure 8
The map of the GOI sequence has the typical anti CD19 CAR [1] sequence with additional IRES EGFP sequence.It is almost twice the length of the original anti CD19 CAR sequence.). Cell screening and process optimization are similar to hPGK-luciferase-IRES-EGFP. Results are shown in Figure 8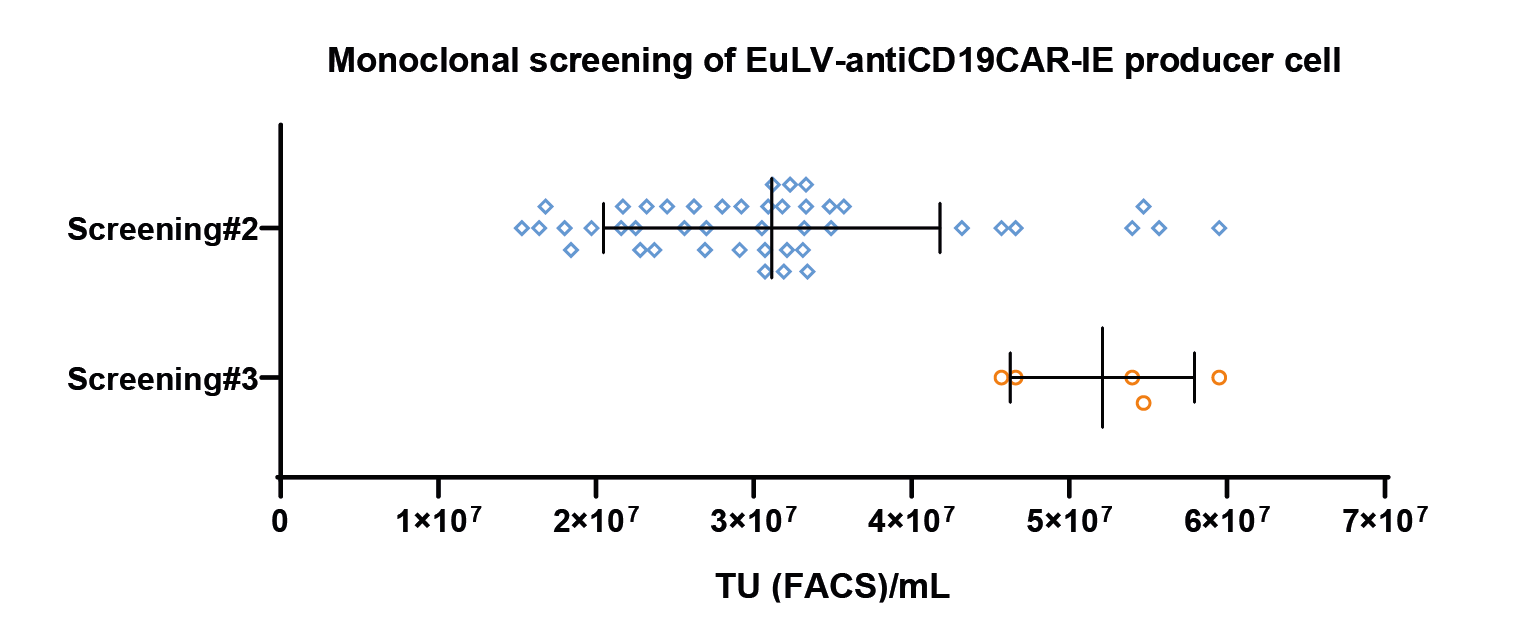 The monoclonal screening result.The x-axis represents the viral titer measured by FACS, representing the 2nd and 3rd screening results. Each dot represents a transduction titer result from one single clone. After 3 rounds of screening, the best clone can reach 5 107 TU/mL measured by FACS. & Figure 9
The monoclonal screening result.The x-axis represents the viral titer measured by FACS, representing the 2nd and 3rd screening results. Each dot represents a transduction titer result from one single clone. After 3 rounds of screening, the best clone can reach 5 107 TU/mL measured by FACS. & Figure 9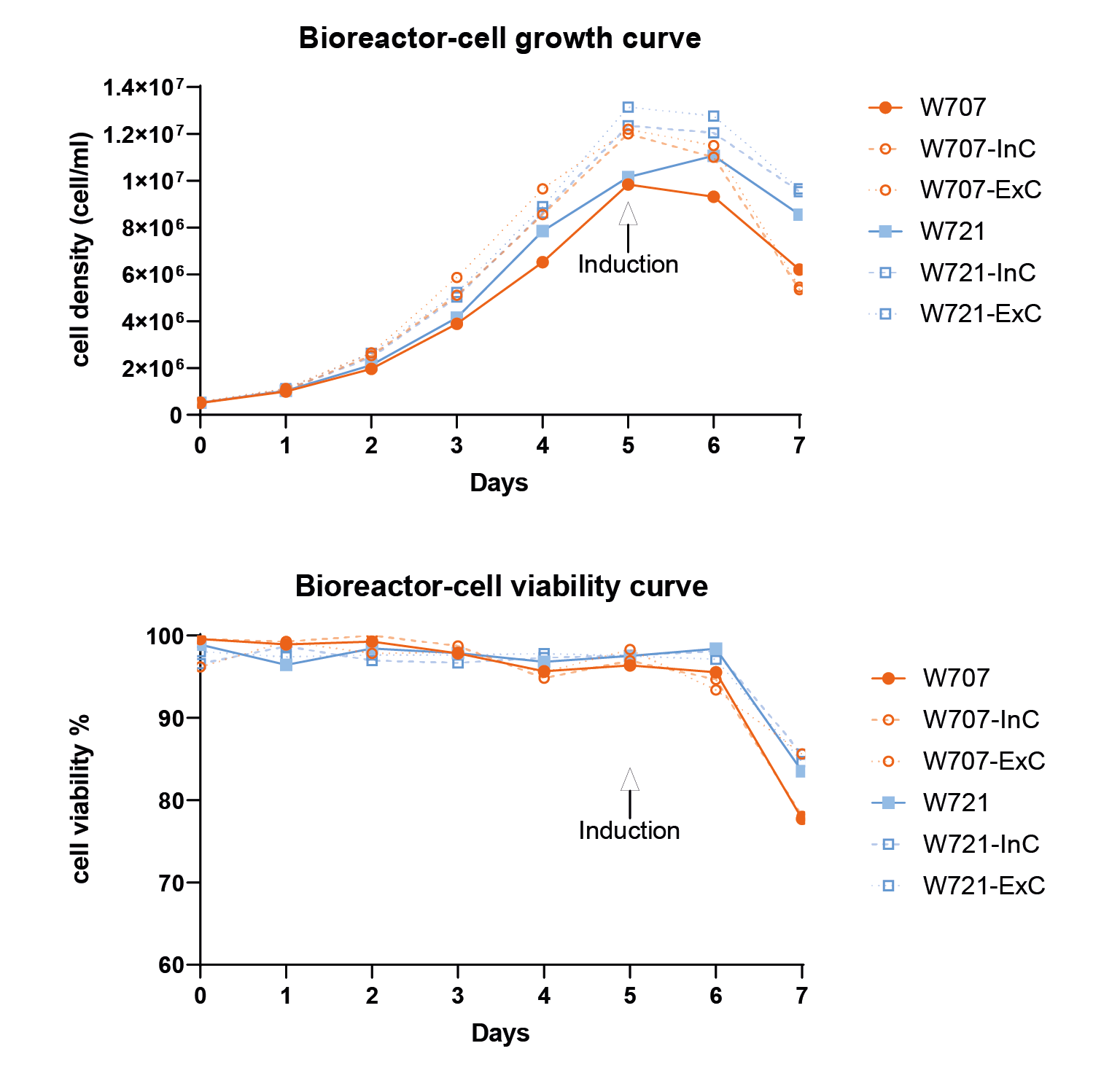 Cell density and cell viability data in 1L WAVE bioreactor.Similar to flasks, they are induced on day 5 and harvested after 48 hours..
Cell density and cell viability data in 1L WAVE bioreactor.Similar to flasks, they are induced on day 5 and harvested after 48 hours..
For this batch of experiment, we get 8.13E10 TU per litre in the culture medium and 2.91E10 TU per litre after purification. From the result of HPLC analysis, the viral particle purity is 84.5%. The manufacturing process is still under optimization (Table 2).
| Table 2. First, the lentivirus is removed by centrifugation or depth filtration, and then by 0.45-micron filtration to further remove impurities such as cell debris (step 1) and benzonase digestion (step 2). | ||||
| Process flow | Purity % | TU titer 1E07Tu(RLU)/ml | Recovery (TU based %) | Inducer residue |
| Medium | N/A | 8.13 | 100 | N/A |
| Step 1 | 0.16 | 7.74 | 80.14 | N/A |
| Step 3 | 1.38 | 5.94 | 76.16 | N/A |
| Step 4 | 34.4 | 7.61 | 49.85 | N/A |
| Step 6 | 84.5 | 78.6 | 35.78 | Not detected |
| Then, the purified lentivirus was obtained by ion-exchange chromatography (step 3), gel filtration chromatography (step 4), ultrafiltration concentration washing (step 5), and sterile filtration (step 6). | ||||
Other GOIs
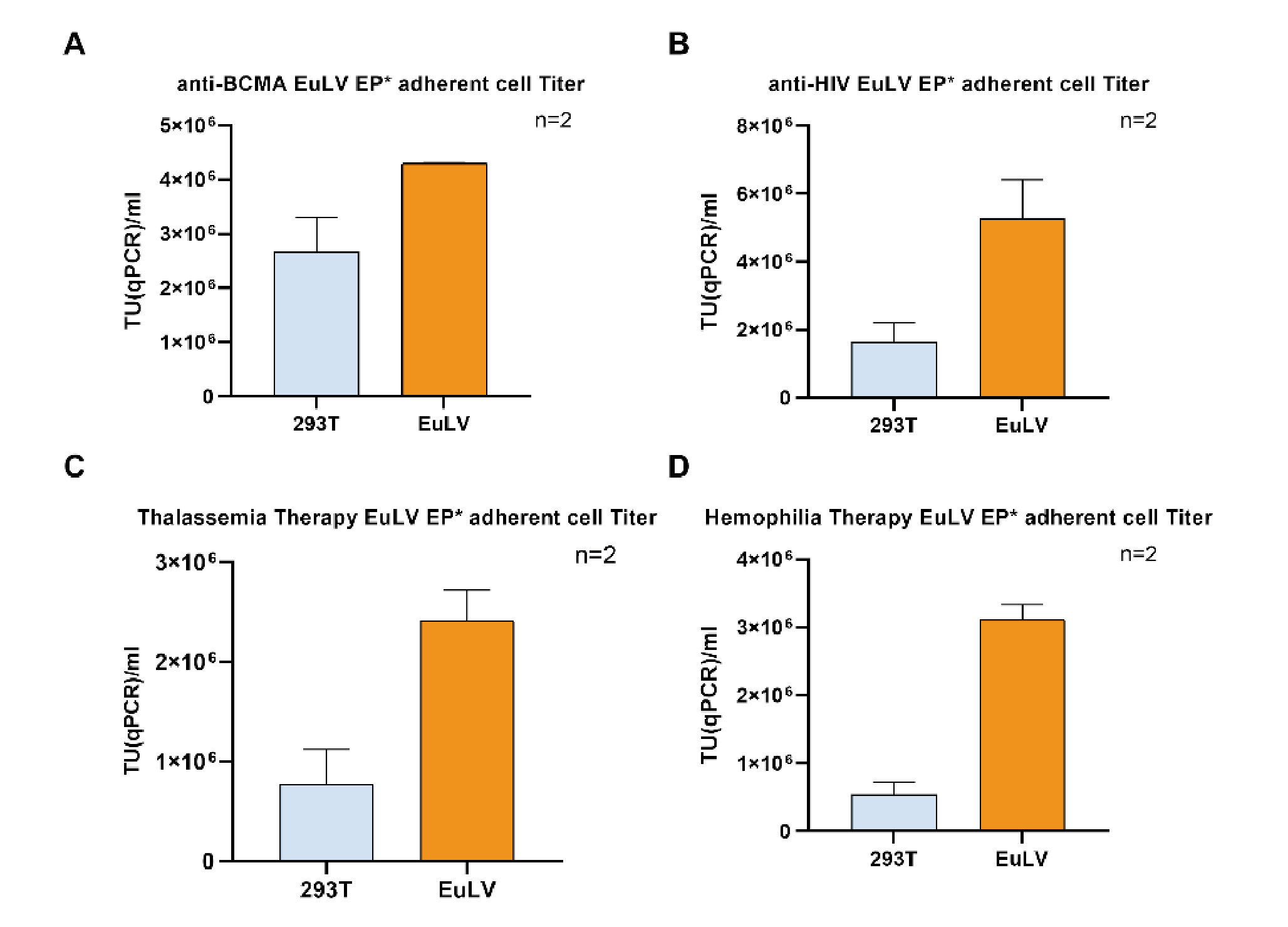
In order to test the versatility of the EuLV® system, the other four GOIs were also used to construct producer cell lines through packaging cells, and the lentiviral yield was tested under adherent conditions. The structures of each GOI are shown in Figure 10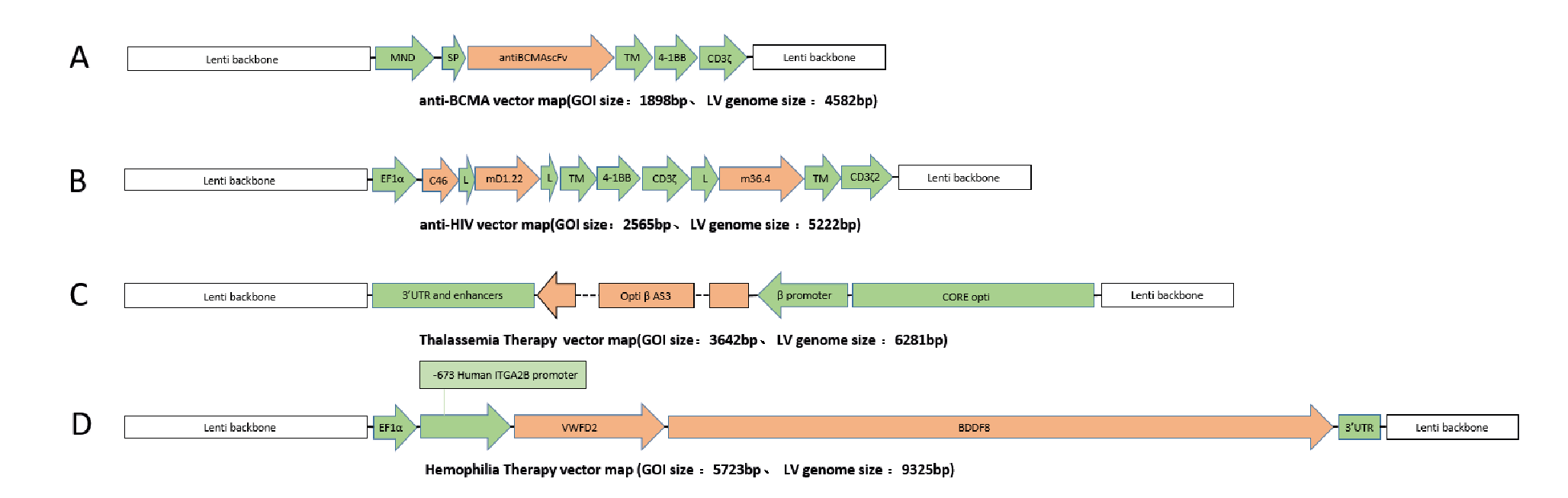 Structures of 4 GOI, A:anti-BCMA vector [2], B:anti-HIV vector [3], C:Thalassemia Therapy vector [4], D:Temophilia Therapy vector [5]., and the detection results of lentiviral infection titres are shown in Figure 11
Structures of 4 GOI, A:anti-BCMA vector [2], B:anti-HIV vector [3], C:Thalassemia Therapy vector [4], D:Temophilia Therapy vector [5]., and the detection results of lentiviral infection titres are shown in Figure 11 During this study, the transduction titer of the enriched pool (EP*) of adherent EuLV producer cells (EuLV ) is measured against the transient transfection method viral yield (293T).The viral titer is higher than the transient method. Since only EP* adherent cells are used for comparison, the viral yield will be improved over 10- fold after cell single clone screening and suspension adaption..
During this study, the transduction titer of the enriched pool (EP*) of adherent EuLV producer cells (EuLV ) is measured against the transient transfection method viral yield (293T).The viral titer is higher than the transient method. Since only EP* adherent cells are used for comparison, the viral yield will be improved over 10- fold after cell single clone screening and suspension adaption..
Discussion
Developing stable lentiviral vector-producing cell lines is time-consuming and labour-intensive complex systems engineering that takes a year or more to fully develop and characterize cell line platforms. Due to the complexity of the work, much published work ultimately failed to meet the needs of the industry due to issues such as titre, cell line stability, and culture adaptation. However, as compensation, once a stable virus producer cell line is successfully developed, the technology has irreplaceable advantages in the field of clinical and industrial applications, the stable producer cell line is more efficient than transient transfection production in terms of process reliability, scale-up capability, production cost, and virus product safety. First, the stable producer cell line production process is more stable and can provide a fully characterized production platform to produce safer viral vectors with low batch-to-batch variability; second, the process is easier to scale up, and the titre without drops rapidly problem when the culture volume increases; in addition, without the addition of raw materials such as DNA plasmids and transfection reagents, the company does not need to establish an additional GMP production line for producing plasmids; finally, it has the higher unit yield and simpler production process quality control. When expanding the production scale, the production process of stable production cell line will further highlight its advantages in R&D, production, management, operation and maintenance, and cost. These advantages are beneficial to the promotion of technology and drug industrialization in the field of causative therapy and cell therapy.
Stable producer cell line for lentiviral vector production
EurekaBio provides CRO services of the EuLV® system. Customers only need to provide gene sequences or plasmids, and EurekaBio will deliver the corresponding monoclonal cell line within 4 months (Figure 12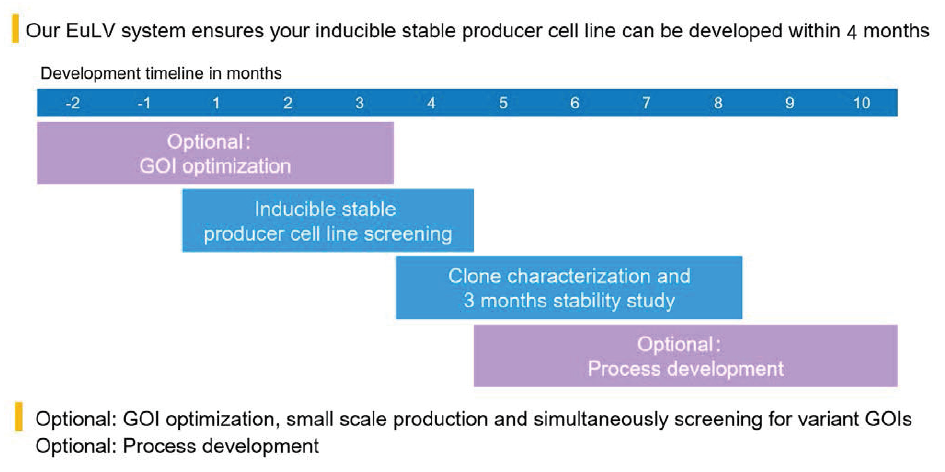 EuLV CRO project development timeline.). Other optional services are also provided, including GOI optimization, clone identification, stability research, upstream and downstream process development.
EuLV CRO project development timeline.). Other optional services are also provided, including GOI optimization, clone identification, stability research, upstream and downstream process development.
References
1.Brogdon J, June C H, Loew A, et al. Treatment of cancer using humanized anti-CD19 chimeric antigen receptor: U.S. Patent 10,221,245[P]. 2019; 3–5. Crossref
2.Friedman KM, Garrett TE, Evans J W et al. (2018). Effective Targeting of Multiple B-Cell Maturation Antigen– Expressing Hematological Malignances by Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor T Cells. Hum. Gen. Ther. 2018; 29; 585–601. Crossref
3.Anthony-Gonda K, Bardhi A, Ray A et al. Multispecific anti-HIV duoCAR-T cells display broad in vitro antiviral activity and potent in vivo elimination of HIV-infected cells in a humanized mouse model. Sci. Trans. Med. 2019; 11. Crossref
4.Kohn D B, Morgan R A, Hollis R P. Optimized lentiviral vector for stem cell gene therapy of hemoglobinopathies: U.S. Patent Application 16/466,970[P]. 2020; 4–9 Crossref
5.Du LM, Nurden P, Nurden AT et al. Platelet-targeted gene therapy with human factor VIII establishes haemostasis in dogs with hemophilia A. Nat Commun. 2013; 4, 2773 Crossref
Affiliations
Bofu Xue
Shenzhen Eureka Biotechnology Co., Limited
Nathan Yang
Shenzhen Eureka Biotechnology Co., Limited
Amon Liu
Shenzhen Eureka Biotechnology Co., Limited
Bright Liu
Shenzhen Eureka Biotechnology Co., Limited
Jessie Liu
Shenzhen Eureka Biotechnology Co., Limited
Aaron Lin
Shenzhen Eureka Biotechnology Co., Limited
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: The authors have no conflicts of interest.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2022 Shenzhen Eureka Biotechnology Co., Limited. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Jan 10 2022; Revised manuscript received: Feb 17 2022; Publication date: Mar 21 2022.


![Structures of 4 GOI, A:anti-BCMA vector <b>[2]</b>, B:anti-HIV vector <b>[3]</b>, C:Thalassemia Therapy vector <b>[4]</b>, D:Temophilia Therapy vector [5].](https://cdn.insights.bio/uploads/Figure/C_EUR_003Fig10174.png)