Clarification of recombinant adeno-associated virus (rAAV) & lentivirus from adherent culture
Cell & Gene Therapy Insights 2022; 8(3), 483 –493
DOI: 10.18609/cgti.2022.070
In recent years the cell and gene therapy industries have been rapidly expanding, with two of the most utilized viral vector classes being adeno-associated virus (AAV) and lentivirus. With clinical success comes the need to develop and scale-up efficient manufacturing processes. As both of these vectors are produced in cells, the first step in their purification is to clarify them from the cell culture. There are many technologies traditionally used for cell culture clarification but given the projected manufacturing scales and need for single-use consumables a combination of depth and membrane filtration is a logical fit for batch processing of viral vectors. This work focuses on developing filtration-based clarification processes for both AAV and lentivirus. The data shows robust turbidity reduction and step yields across batches, scales, and AAV serotypes. We discuss how capacity can be impacted by feedstream characteristics and how capacities translate to manufacturing footprints. Finally, we discuss some process considerations that are unique to viral vector processing and critical to successful vector harvest.
In recent years, development in the gene therapy industry has grown rapidly [1]Barlow JF, Tredenick-Fricke TL, Glover C, Hitchcock T, King D, Krishnan M, et al. Insights on Successful Gene Therapy Manufacturing and Commercialization. 2020. 1–44. https://go.pall.com/genetherapyebook.html?utm_source=cellculturedish&utm_medium=social&utm_campaign=20-10-121-GTEBO&utm_term=ebook&utm_content=cellculturedish, [2]Forsber N, King D, Glover C, Madsen J, Hughes J V, Moore A, et al. Key Considerations in Gene Therapy Manufacturing for Commercialization. Cell Cult. Dish. 2018; . As of 2022 there are over 20 gene and gene-modified cell therapies approved by regulatory bodies across the world with hundreds more in clinical trials. The two largest classes of viral vectors in development today are recombinant adeno-associated virus (AAV) and lentivirus [3–5]. AAV is a non-enveloped virus ~20 nm in diameter. The recombinant vector can package ~4.7 kilobases of DNA and shows relatively low immune response compared to retroviruses and adenoviruses [6]Rabinowitz J, Chan YK, Samulski RJ. Adeno-associated Virus (AAV) versus immune response. Viruses 2019; 11(2), 1–11. . Furthermore, it is relatively stable under standard bioprocessing conditions [7]Rayaprolu V, Kruse S, Kant R et al. Comparative Analysis of Adeno-Associated Virus Capsid Stability and Dynamics. J. Virol. 2013; 87(24), 13150–60. , [8]Bennett A, Patel S, Mietzsch M et al. Thermal Stability as a Determinant of AAV Serotype Identity. Mol. Ther. - Methods Clin. Dev. 2017; 6, 171–82. . Lentiviral vectors are enveloped and are ~120 nm in diameter. They deliver an RNA payload and are used in both gene therapy as well as many chimeric antigen receptor (CAR) T cell-based cell therapies [9]de las Mercedes Segura M, Kamen A, Garnier A. Downstream processing of oncoretroviral and lentiviral gene therapy vectors. Biotechnol Adv. 2006; 24(3), 321–37. .
Both vectors are expressed from host cells which are often grown on substrates in adherent cell culture bioreactors. In typical rAAV production the product can be found both intra- and extracellularly, and many processes therefore include a cell lysis step to maximize product recovery. In contrast, the majority of lentiviral vectors are secreted from the host cells and can therefore be harvested from the bioreactor supernatant without cell detachment or lysis. Similar to recombinant protein processing, once the crude harvest is collected from the bioreactor the next step is to clarify the product from the complex mixture of insoluble impurities which can include cell debris and any precipitated host-cell protein and DNA.
A typical filtration-based clarification step will include a membrane filter with a thin structure and tight pore rating down to 0.2 mm or in some cases 0.45 mm. This filter is responsible for bioburden removal and some additional particulate removal to protect subsequent purification technologies from fouling. In many cases the membrane filter is preceded by a prefilter with a thicker structure and wider pore range. These can dramatically improve capacity on the membrane filter translating to improved overall process economics and footprint. Depth filters made of a mixture of cellulose, inorganic filter aids, and resins are commonly used prefilters and in addition to the particulate removal can provide some soluble impurity removal through adsorption. Both depth and membrane filters have a long history of use in the biopharmaceutical industry and offer a robust, cost-effective solution for clarification over a wide range of scales [10–12]. However, there are many filter options across the industry ranging in materials of construction, structure, pore size, and available formats which must be considered against the feedstream and product characteristics. AAV and lentivirus have been shown to carry a negative surface charge [8]Bennett A, Patel S, Mietzsch M et al. Thermal Stability as a Determinant of AAV Serotype Identity. Mol. Ther. - Methods Clin. Dev. 2017; 6, 171–82. , [13]Kumru OS, Wang Y, Gombotz CWR et al. Physical Characterization and Stabilization of a Lentiviral Vector Against Adsorption and Freeze-Thaw. J. Pharm. Sci. 2018; 107(11), 2764–74. , but there can be slight differences based on serotype [14]Potter M, Lins B, Mietzsch M et al. A simplified purification protocol for recombinant adeno-associated virus vectors. Mol. Ther. - Methods Clin. Dev. 2014; 1, 14034. , [15]Nam H-J, Lane MD, Padron E et al. Structure of Adeno-Associated Virus Serotype 8, a Gene Therapy Vector. J. Virol. 2007; 81(22): 12260–71. . Depth filters carry a mix of both positive and negatively charged surfaces [16]Khanal O, Singh N, Traylor SJ et al. Contributions of depth filter components to protein adsorption in bioprocessing. Biotechnol. Bioeng. 2018; 115(8), 1938–48. , [17]Nejatishahidein N, Borujeni EE, Roush DJ, Zydney AL. Effectiveness of Host Cell Protein Removal using Depth Filtration with a Filter Containing Diatomaceous Earth. Biotechnol. Prog. 2020; 36(6), e3028. , and of course any charge interaction will be dependent on the ionic strength of the spent media and process buffers. There is some evidence showing lentivirus will bind to diatomaceous earth, a filter aid used in many depth filters [18]Labisch JJ, Bollmann F, Wolff MW, Pflanz K. A new simplified clarification approach for lentiviral vectors using diatomaceous earth improves throughput and safe handling. J. Biotechnol. 2021; 326, 11–20.. Finding the optimal set of filters for clarification remains a challenge for these emerging fields.
In this work we evaluate filtration-based clarification options for recombinant AAV and lentivirus coming from adherent cell culture. The data demonstrates that depth filtration combined with membrane filter clarification can be an effective solution for viral vector manufacturing and provides some guidance in filter selection and screening. Finally, we discuss some subtle differences in filter options to consider during process development to improve the chances of successful scale-up, tech transfer, and production.
Materials & Methods
Crude harvest supply
All recombinant AAV5 used in this work was supplied through transient transfection of HEK293T cells using a PEIpro® transfection reagent (Polyplus-transfection). For transfection, plasmids pCDAAV-Helper, pCDAAV-CMV-eGFP, and pCDAAV5-R/C (Creative Biolabs) were used in a 1:1:1 ratio. A DNA:PEIpro ratio of 1:1 was used for transfection. Adherent cultures were either produced with Corning® CellSTACK® chambers or in Pall's iCELLis® Nano bioreactors. Following transfection the cultures were grown for 5 days at which point the culture supernatant was removed, the cells were lysed using a detergent buffer (10 mM Tris (pH 8.0), 160 mM NaCl, 2 mM MgCl2, 1% Tween 20), and the lysate collected from the bioreactor. The supernatant and lysate were treated with an endonuclease (25 U/mL) and the total NaCl concentration was increased to 500 mM prior to clarification. rAAV5 concentration in the crude harvest averaged 7.4 × 109 with a 95% confidence interval of
± 2.0 × 109 gene copies per milliliter (gc/mL). HEK293 cells producing AAV8 and AAV9 vectors encapsulating a green fluorescent protein (GFP) reporter gene were procured from Vector BioLabs. The cells were grown in adherent CellSTACK chambers and contained the expressed vectors intracellularly upon arrival. Treatment of the cells was designed to mimic that of the AAV5 harvest from the iCELLis Nano bioreactors. Cells were lysed with the same detergent buffer as described above, diluted into culture medium, and endonuclease-treated prior to clarification. Concentrations for AAV8 and AAV9 crude harvests were 1.4 × 108 and 1.7 × 108 gc/mL, respectively.
All lentivirus pools used in this study were produced by transfection of adherent HEK293T cells grown in CellSTACK chambers or iCELLis Nano bioreactors. The Lentivirus produced was HIV-1 derived with a VSV-G pseudotype carrying a gene for GFP. Lentivirus plasmids were purchased from Aldevron and used in a ratio of 2 pALD-VSV-G-A:2.5 pALD-GagPol-A:1 pALD-Rev-A:2.5 pALD-LentiEGFP-A. A DNA:PEIpro ratio of 1:2.75 was used for transfection. Supernatants were collected from the bioreactor 48–72 h after transfection, 2 mM MgCl2 was added, and the pool was endonuclease treated (25 U/mL). Lentivirus concentration in the crude harvest averaged 7.1 × 107 with a 95% confidence interval of ± 4.9 × 107 infectious particle per milliliter (IP/mL).
Filtration
A description of the prefilters and membrane filters used in this work is summarized in Table 1. Pall’s PreFlow™ UB media is made of resin-bonded glass fiber and provides a gamma-stable option for protecting membrane filters in bioprocessing. In this work 47 mm discs were tested using stainless steel holders (effective filter area (EFA) = 11.1 cm2). Seitz™ P-series depth filter sheets are made up of a combination of cellulose, inorganic filter aids, and a binding resin. The V100P is a sheet designed specifically for processing viral vectors that is low-charge and free of diatomaceous earth. The PDK11 filter is a dual-layer filter made up of the same V100P sheet on the bottom and a K900P sheet on top. The K900P media is a standard grade in Pall’s Seitz P-series depth filter line made of cellulose, filter aids, and resin with a retention rating of 8–20 mm. For screening, the V100P and PDK11 filters were tested in Supracap™ 50 capsules (EFA = 22 cm2). For larger scale work PDK11 filters were also evaluated in Supracap 100 capsule format (EFA = 0.025 m2 for 127 mm and 0.05 m2 for 254 mm).
| Table 1. Description of prefilters and membrane filters used in this work. | ||||
| Role | Filter media | Materials of construction | Layers | Retention rating |
| Prefiltration | PreFlow UB | Resin-bonded glass fiber | 1 | 0.45 mm |
| Seitz V100P | Cellulose fibers, perlite, and resin | 1 | 2–4 mm | |
| Seitz PDK11 | Cellulose fibers, filter aids, and resin | 2 | 8–20 mm/2–4 mm | |
| Bioburden reduction | Supor EAV | Single-layer polyethersulfone (PES) | 1 | 0.2 mm |
| Sterilizing grade | Supor EKV | Dual-layer PES | 2 | 0.2 mm |
In select studies filtrate pools from a single prefilter was run over Pall’s Supor® EKV sterilizing grade or Supor EAV bioburden reduction filters. These were tested in Mini Kleenpak™ syringe filters (EFA = 2.8 cm2) and Mini Kleenpak 20 capsule (EFA = 20 cm2) formats. For larger scale work the Supor EKV filters were also evaluated in Mini Kleenpak capsule format (EFA = 220 cm2).
All filtration work described here was run at constant flux on PendoTECH Filter Screening System (NFF) control systems with peristaltic pumps on the feed lines. Pressures and filtrate volumes were recorded over time. In all trials filters were equilibrated using a 1× phosphate buffered saline (PBS; pH 7.4) solution at ≥50 L/m2. Prefilter capsules were drained prior to loading process fluid. Experiments used to determine filter capacity were run at constant flux to a terminal pressure of 0.7 bar (10 psi). AAV capacity trials were run at 200 liters/m2/hour (LMH) on the prefilters and 1000 LMH on the membrane filters. Lentivirus capacity trials were run at 200 LMH on the prefilters and 500 LMH on the membrane filters. A post-use buffer chase of 1.5× hold-up volumes was also employed to maximize virus recovery. This flush was pooled with the product filtrate and sampled for
virus titer to determine yields.
Analytics
Pool turbidities were measured offline on a Hach® 2100Q portable turbidimeter. AAV concentrations were measured by a digital droplet polymerase chain reaction (ddPCR) method using the BIORADQX200 AutoDG Droplet Digital PCR System. Non-encapsidated DNA was digested at 37°C for 1 h using an RNAse Free DNase I kit (Qiagen). Once digested, the samples were diluted 1:100 in 1× TE solution (Integrated DNA Technologies), supplemented with Pluronic PF-68 to 0.01% and ddPCR was performed using primers targeting an amplicon in the gene of interest. Lentivirus concentrations were quantified using a flow-cytometry based transduction unit (TU) assay. HEK293 cells were seeded into 24-well plates at 1 × 105 cells/well and incubated overnight at 37°C in 5.0% CO2. Serial two-fold dilutions of samples were performed prior to addition to cells. A spinoculation was then performed for 2 h at 1000 xg at 25°C. Post-spinoculation additional media was added to the wells and the plates were incubated for 48 h at 37°C in 5.0% CO2. The wells were aspirated of media, washed with 1× PBS, aspirated again, and then TrypLE (ThermoFisher) was added to detach the cells for fluorescent cytometric analysis on a CytoFLEX (Beckman Coulter). Step yields were calculated using Equation 1 below where Vf and Vp refer to feed and filtrate pool volumes and Cf and Cp refer to feed and filtrate pool concentrations respectively.

Results
AAV screening
Initial AAV screening work was conducted on the prefilter to identify an appropriate filter train for clarification. The V100P was selected as a single-layer depth filter option as it was specifically designed for the processing of viruses. This filter media contains no diatomaceous earth and a relatively low overall charge. A dual-layer PDK11 depth filter was also evaluated which contains the same V100P sheet with a more open K900P sheet on top. Both filters were evaluated with an adherent AAV5 crude harvest pool measured at 36 Nephelometric Turbidity Unit (NTU) and 9.0 × 109 gc/mL. Capacity was defined through constant flux (PMAX) studies run at 200 LMH to a terminal pressure of 0.7 bar (10 psi). As shown in Figure1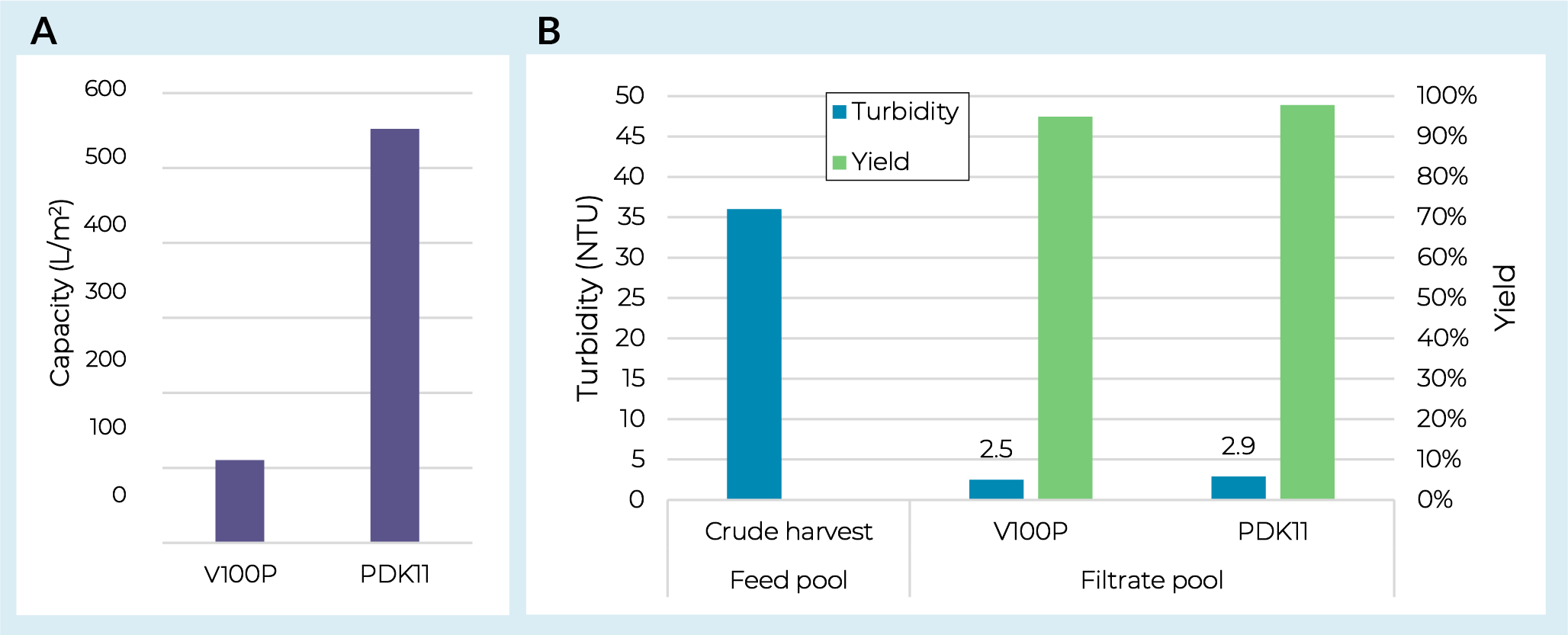 Depth filter screening with AAV5.(A) Capacities for depth filters loaded with AAV5 crude harvest. (B) Pool turbidities and depth filter yields from AAV5 screening. both V100P and PDK11 filters demonstrated high yields (≥95%) and strong turbidity reduction (<3 NTU in the filtered pool). However, with this feedstream there was a significant capacity benefit from the dual-layer PDK11 (Figure 1a), reaching >500 L/m2 at 0.7 bar (10 psi). Both depth-filtered pools were then taken offline and used to measure capacity on Supor EKV sterilizing-grade filters with both showing capacities of >1700 L/m2 and AAV5 yields of >99% (data not shown).
Depth filter screening with AAV5.(A) Capacities for depth filters loaded with AAV5 crude harvest. (B) Pool turbidities and depth filter yields from AAV5 screening. both V100P and PDK11 filters demonstrated high yields (≥95%) and strong turbidity reduction (<3 NTU in the filtered pool). However, with this feedstream there was a significant capacity benefit from the dual-layer PDK11 (Figure 1a), reaching >500 L/m2 at 0.7 bar (10 psi). Both depth-filtered pools were then taken offline and used to measure capacity on Supor EKV sterilizing-grade filters with both showing capacities of >1700 L/m2 and AAV5 yields of >99% (data not shown).
AAV process robustness
& scalability
Turbidity of the crude harvest is often used as a rough measurement to encompass key feedstock characteristics including culture cell density and viability prior to harvest, particle concentration, and particle size distribution. Using AAV5 crude harvest pools produced in iCELLis Nano bioreactors we ran seven replicate trials with feedstocks ranging from 29–133 NTU. As expected, we did generally see higher feed turbidites translate to lower prefilter capacities (consistent with the lentiviral data shown below). However, there was not a consistent trend and we saw one example of a highly turbid feed leading to low fouling on the prefilter and high fouling on the membrane filter. We hypothesize this was due to a difference in particle size distribution and highlights that while turbidity is a useful tool, it is not a comprehensive measure of crude harvest characteristics. Regardless of the crude harvest turbidity, over the seven batches tested capacities were all >250 L/m2 on a PDK11 depth filter and >400 L/m2 on a subsequent Supor EKV filter. The key finding was that across the range of feed turbidities we observed strong robustness for turbidity reduction and yield with pool turbidities at 3.0 ± 1.3 NTU (Figure2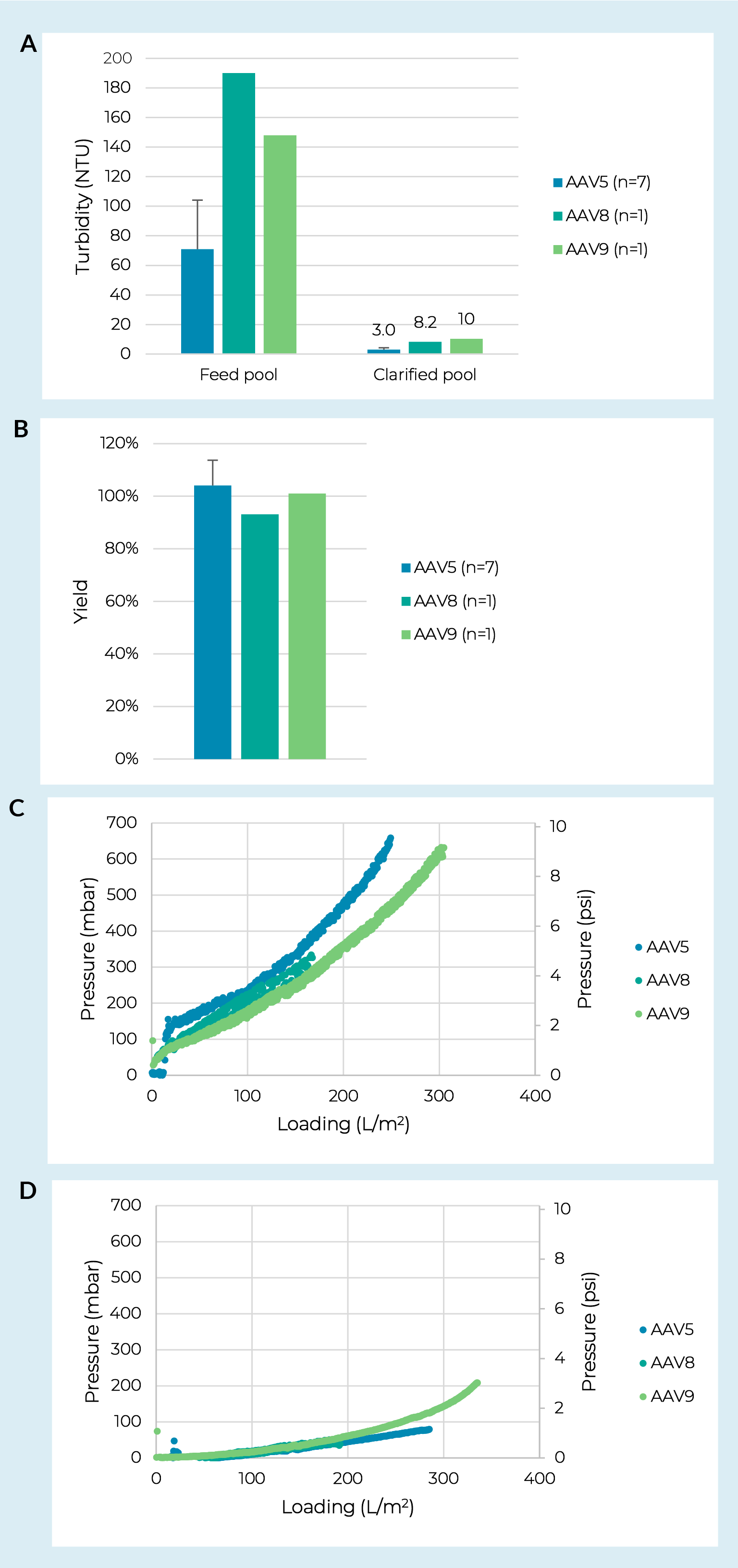 Clarification process robustness against AAV serotype.Turbidity reduction (A) and step yield (B) for clarification of three different AAV serotypes using a combination of PDK11 and Supor EKV filtration. Where multiple trials were run error bars represent a 95% confidence interval. Differential pressure vs. loading curves from PDK11 (C) and Supor EKV filters (D) run with crude harvests of AAV8, AAV9, and a representative batch of AAV5 which had a crude harvest turbidity closest to the other two serotypes (133 NTU).a) and yields at 104% ± 9.6% (Figure 2b).
Clarification process robustness against AAV serotype.Turbidity reduction (A) and step yield (B) for clarification of three different AAV serotypes using a combination of PDK11 and Supor EKV filtration. Where multiple trials were run error bars represent a 95% confidence interval. Differential pressure vs. loading curves from PDK11 (C) and Supor EKV filters (D) run with crude harvests of AAV8, AAV9, and a representative batch of AAV5 which had a crude harvest turbidity closest to the other two serotypes (133 NTU).a) and yields at 104% ± 9.6% (Figure 2b).
Next, we evaluated two additional AAV serotypes (AAV8 and AAV9) produced in adherent culture grown in CellSTACK chambers. Here we saw no significant difference in pressure curves on the PDK11 (Figure 2c) or Supor EKV filter (Figure 2d) when run with an AAV8 or AAV9 feed compared to an AAV5 feed with a similar turbidity. The clarification train showed strong robustness to serotype for turbidity reduction and yield with all clarified pools at or below 10 NTU
(Figure 2a ) and yields >93% (Figure 2b).
Assessing scalability is another critical step in the development of a clarification process. Using the adherent AAV5 material, performance of the PDK11 + Supor EKV filter train was evaluated across process development and pilot-scale capsules. Throughputs ranged from 180 to 550 L/m2 on the depth filters and 300 to 1900 L/m2 on the sterile filters. Note that in most cases the entire batch was processed before reaching capacity on either filter and that the different scales were tested with independent feedstocks making it difficult to comment on scalability of filter capacity. Pool turbidities and AAV yields are shown in Figure3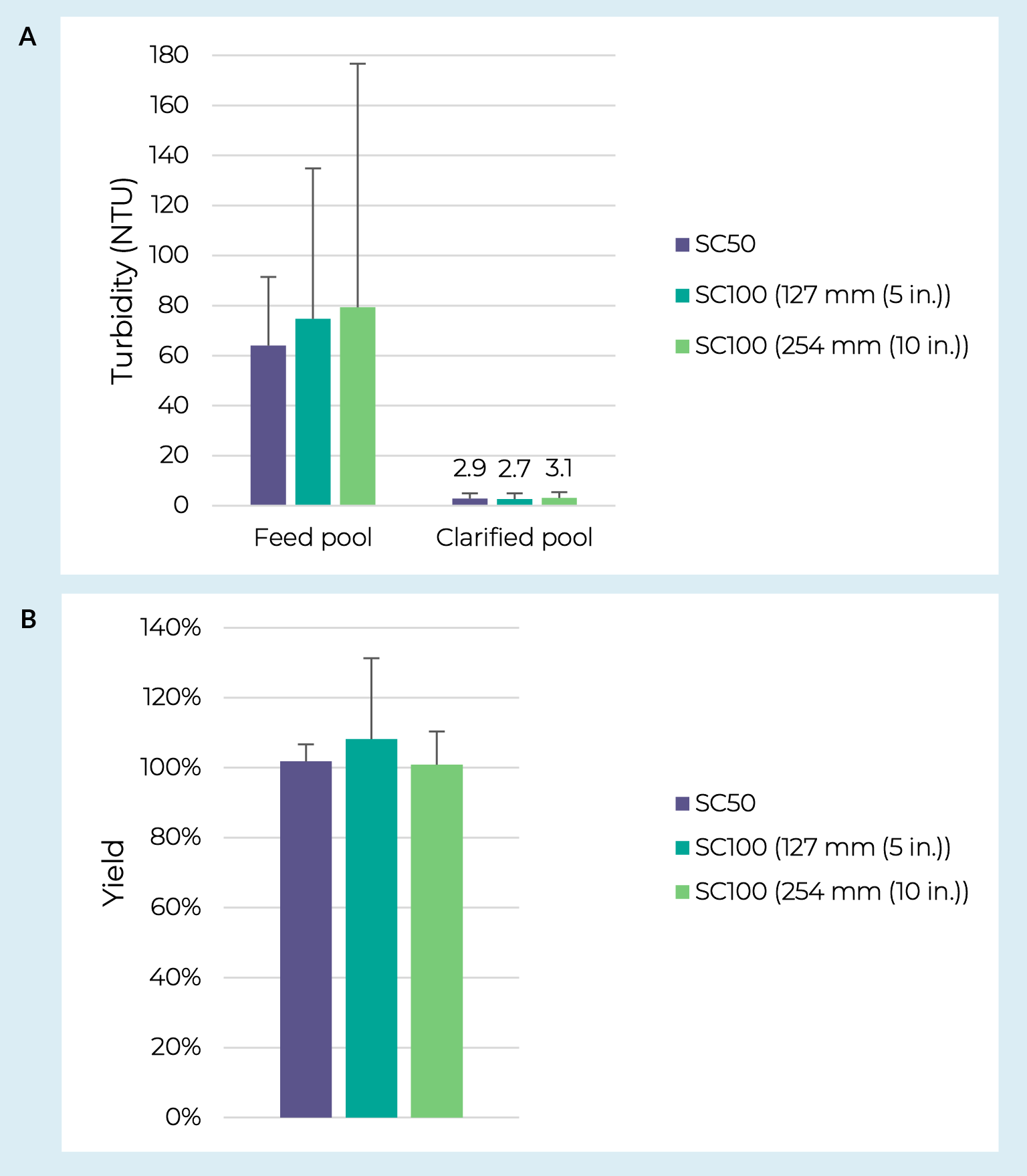 AAV clarification scalability.Turbidity reduction (A) and yields (B) from AAV5 crude harvest clarified over a range of depth and sterile filter scales. SC50 indicates PDK11 Supracap 50 depth filters run over Supor EKV media in Mini Kleenpak syringe filters or Mini Kleenpak 20 filter capsules (n = 3). SC100 indicates PDK11 Supracap 100 depth filters run over Supor EKV media in Mini Kleenpak filter capsules (n = 3 for 127 mm; n = 2 for 254 mm). Error bars represent a 95% confidence interval. between the development-scale PDK11 in Supracap 50 capsules + Supor EKV membrane in Mini Kleenpak syringe filters or Mini Kleenpak 20 capsules and the pilot-scale PDK11 in Supracap 100 capsules + Supor EKV filters in Mini Kleenpak capsules. Critically, there was no statistically significant difference observed between scales for pool turbidity or AAV yield (p > 0.05 from two-sample T-tests).
AAV clarification scalability.Turbidity reduction (A) and yields (B) from AAV5 crude harvest clarified over a range of depth and sterile filter scales. SC50 indicates PDK11 Supracap 50 depth filters run over Supor EKV media in Mini Kleenpak syringe filters or Mini Kleenpak 20 filter capsules (n = 3). SC100 indicates PDK11 Supracap 100 depth filters run over Supor EKV media in Mini Kleenpak filter capsules (n = 3 for 127 mm; n = 2 for 254 mm). Error bars represent a 95% confidence interval. between the development-scale PDK11 in Supracap 50 capsules + Supor EKV membrane in Mini Kleenpak syringe filters or Mini Kleenpak 20 capsules and the pilot-scale PDK11 in Supracap 100 capsules + Supor EKV filters in Mini Kleenpak capsules. Critically, there was no statistically significant difference observed between scales for pool turbidity or AAV yield (p > 0.05 from two-sample T-tests).
Lentivirus screening
Lentiviral work started with prefilter screening using an adherent crude harvest pool taken from an iCELLis Nano bioreactor with a turbidity of 38.5 NTU and lentivirus concentration of 3.7 × 107 IP/mL. Previous data has demonstrated successful clarification of lentivirus from adherent cultures using various combinations of glass fiber prefilters and PES or PVDF membrane filters [19]Raghavan B, Collins M, Walls S, Lambropoulos A, Bergheim-Pietza S. Development of gene therapy purification processes. Cell Gene Ther. Insights. 2019; 5(9), 1311–22. , [20]Moreira AS, Faria TQ, Oliveira JG, Kavara A, Schofield M, Sanderson T, et al. Enhancing the purification of Lentiviral vectors for clinical applications. Sep. Purif. Technol. 2021; 274, 118598.. Using crude harvest pools with turbidities of approximately 10 NTU these synthetic filter options provided capacities of >1500 L/m2 and infectious particle recoveries of >75%. The PreFlow UB resin-bonded glass fiber filter was therefore included in this screening. Due to a significantly higher crude harvest turbidity in this work the V100P and PDK11 depth filters were also included. Lentivirus crude harvest was loaded onto all prefilters at a constant flux of 200 LMH to a terminal pressure of 0.7 bar (10 psi). The data revealed similar capacities for the PreFlow UB and V100P prefilters at approximately 250 L/m2 whereas the PDK11 provided an approximately four-fold higher capacity, achieving 1000 L/m2 (Figure4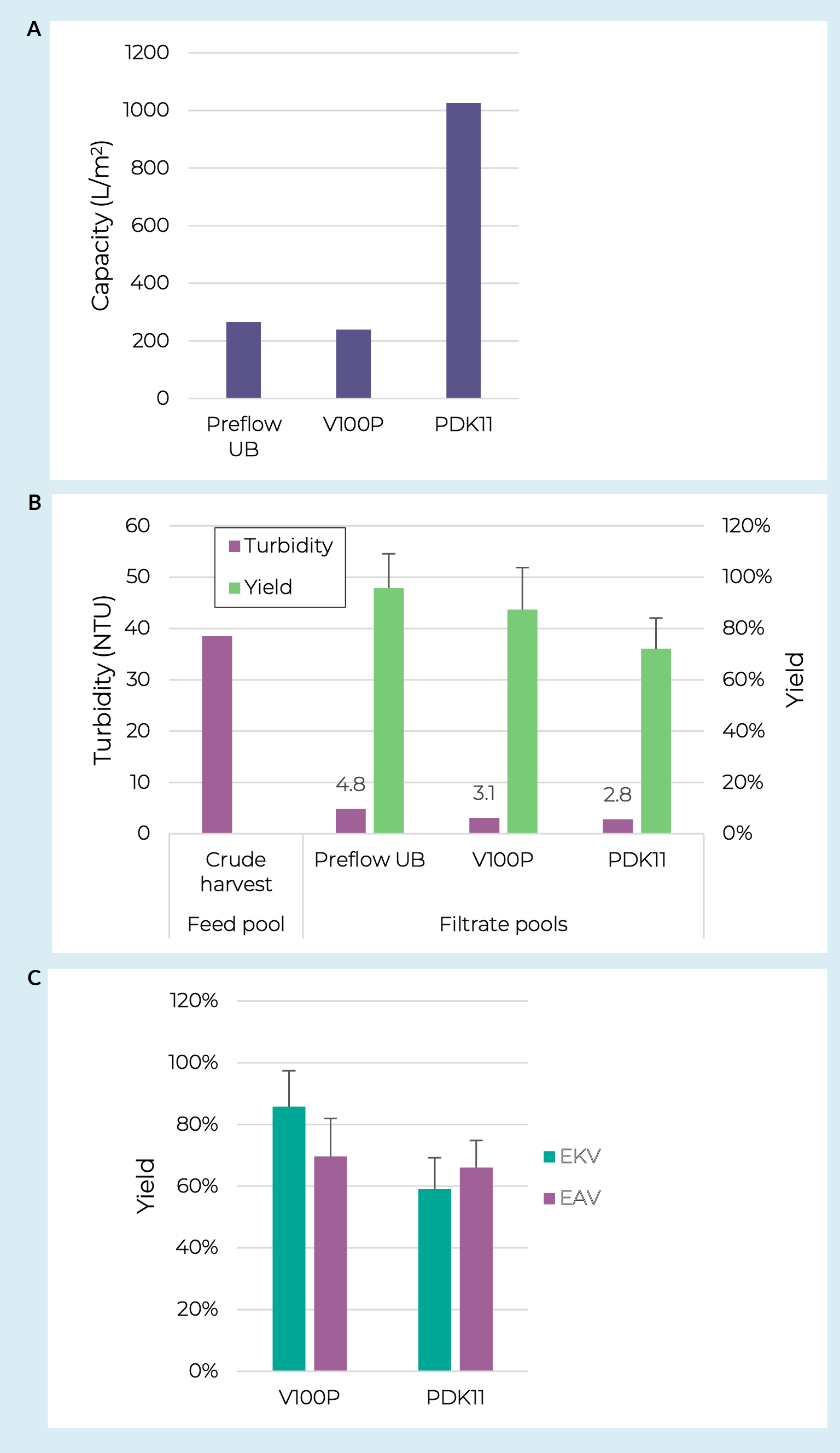 Depth filter and sterile filter screening with lentivirus.A & B. Filter capacity, pool turbidity, and step yields for lentiviral crude harvests processed over three different prefilters. C. Lentiviral step yields over the full clarification (prefilter + membrane filter) for four different filter combinations. Error bars represent 95% confidence intervals based off TU assay technical replicates.a).
Depth filter and sterile filter screening with lentivirus.A & B. Filter capacity, pool turbidity, and step yields for lentiviral crude harvests processed over three different prefilters. C. Lentiviral step yields over the full clarification (prefilter + membrane filter) for four different filter combinations. Error bars represent 95% confidence intervals based off TU assay technical replicates.a).
While all three prefilters reduced the turbidity below 5 NTU, the cellulose-based filters did show slightly lower turbidity levels than the PreFlow UB prefilter (Figure 4b). This turbidity difference correlated to capacity differences on the downstream membrane filters. Pools from each prefilter were run over Supor EKV and Supor EAV filters in parallel. The capacities for those loaded with PreFlow UB filtrate were 34 and 32 L/m2 respectively. In contrast, the cellulose depth filtered pools led to membrane filter capacities between 390 and 480 L/m2.
The cellulose depth filter + membrane filter combinations were evaluated for lentiviral step yields (Figure 4c). The V100P combinations appeared to have slightly higher yields than the PDK11 combinations. This could be expected as the dual-layer PDK11 does contain some diatomaceous earth which has been previously shown to reduce filtrate lentivirus levels [18]Labisch JJ, Bollmann F, Wolff MW, Pflanz K. A new simplified clarification approach for lentiviral vectors using diatomaceous earth improves throughput and safe handling. J. Biotechnol. 2021; 326, 11–20., however more replicates will need to be run to determine if the difference is real and reproducible. Regarding the membrane filters, we did not observe a clear benefit for capacity or yield between the two tested here.
Lentivirus process robustness
Based on the balance between yield and capacity the V100P prefilter was selected for additional testing. The Supor EKV filter was selected as the membrane filter as it provides a sterile filtrate stream and showed no drop-off in capacity or yield. Two additional batches of adherent lentivirus crude harvest from CellSTACK chambers were processed over the V100P + Supor EKV filters. Over the three runs feed turbidity ranged from 20.2–72.9 NTU, feed concentration ranged from 3.7 × 107–1.2 × 108 IP/mL, and the step yield over the depth and membrane filters averaged 74%. Note we did not observe any clear trend between feed turbidity and yield. Prefilter pressure vs. loading curves are presented in Figure5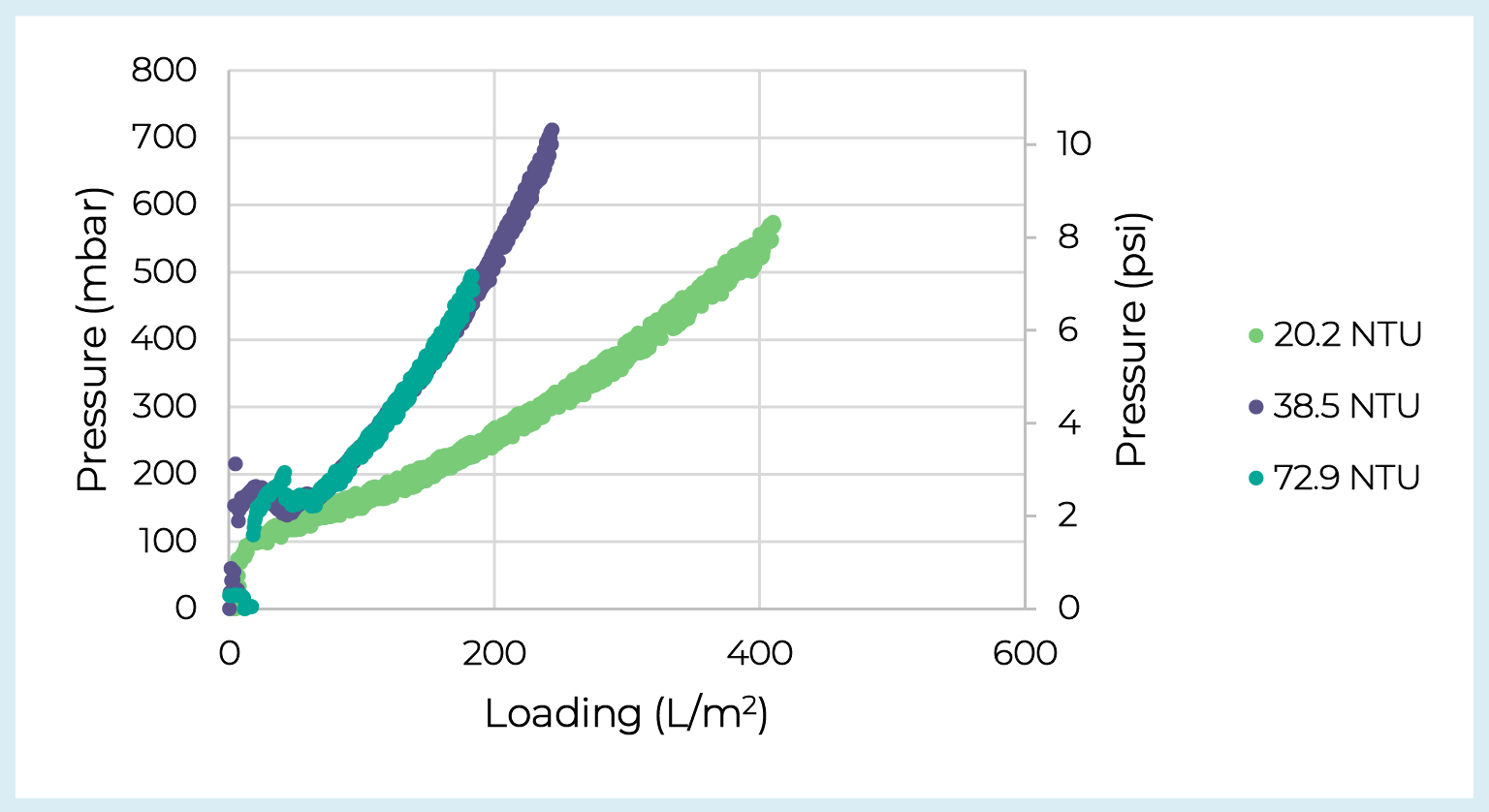 Depth filter capacity across lentivirus batches.Pressure drop vs. loading for V100P prefilters loaded with three batches of adherent lentivirus crude harvest varying in turbidity. and reveal how feed turbidity can impact depth filter capacity. However, despite the range in crude harvest turbidities, the clarified pools showed consistently low turbidity averaging 2.7 ± 0.6 NTU.
Depth filter capacity across lentivirus batches.Pressure drop vs. loading for V100P prefilters loaded with three batches of adherent lentivirus crude harvest varying in turbidity. and reveal how feed turbidity can impact depth filter capacity. However, despite the range in crude harvest turbidities, the clarified pools showed consistently low turbidity averaging 2.7 ± 0.6 NTU.
Discussion
The work described here provides a case study for clarification process development of adherent lentivirus and AAV cultures. For both viral vector classes we found filter combinations that could provide consistently low filtrate turbidities (≤10 NTU) despite relatively large variance in feedstream turbidities. The feedstream turbidity range observed here is likely an extreme case as the cell culture process was being developed in parallel with this clarification work. However, even in tightly controlled processes there is some variability in crude harvest characteristics known to impact clarification such as cell density, viability, particle concentration, and particle size distribution. Therefore, the crude harvest variability tested here provided a nice challenge for assessing process robustness. Step yields also showed strong consistency, particularly for AAV which averaged 103 ± 7.7% across all batches, serotypes, and scales. This consistency could make a depth + membrane filter harvest process fit into a platform to be used across an AAV product portfolio.
While the data shared here should provide some guidance for process development, the optimal filter train will depend on many factors including product quality, process economics, and facility footprint constraints. The lentivirus data presented previously [19]Raghavan B, Collins M, Walls S, Lambropoulos A, Bergheim-Pietza S. Development of gene therapy purification processes. Cell Gene Ther. Insights. 2019; 5(9), 1311–22. and here provides a nice case study of the trade-offs. Take for example, an iCELLis 500+ bioreactor with a 10 cm bed that produces ~570 L of crude harvest. If the feed turbidity is <15 NTU it could be possible to get >1000 L/ m2 through a Preflow UB + Fluorodyne® II DBL filters. This would translate to a single 254 mm (10 in.) PreFlow UB filter capsule and a single 254mm (10 in.) Fluorodyne II DBL filter capsule with the important benefit of both being available in closed and presterilized assemblies. However, with feed turbidites tested in this work, a similar filter train would need to run six 762 mm (30 in.) membrane filters in parallel which would be logistically challenging. In this case the cellulose depth filter options may be needed to simplify the process and reduce the footprint down to a single Stax™ capsule and one 508 mm (20 in.) membrane capsule. Note that these estimates do not include a safety factor which should be included [21]Lutz H. Rationally defined safety factors for filter sizing. J Memb Sci. 2009;341(1–2), 268–78., but nonetheless illustrate how capsule formats and manufacturing-scale can help define the optimal process.
Another interesting example of process trade-offs was seen in the lentiviral membrane filter comparison. While we did not observe a significant difference between the Supor EKV and Supor EAV filters in terms of capacity, pool turbidity, or yield, they each carry unique process benefits. The Supor EKV filter is a sterilizing-grade filter which may allow for more flexibility in pool hold time. However, because the Supor EAV bioburden-reduction filter incorporates a single-layer membrane it can hold more filter area per capsule, and therefore can have some footprint benefit over the Supor EKV filter in some situations, whilst still providing a high level of
bioburden reduction assurance.
The long history of success for these depth and membrane filters in the biopharmaceutical industry combined with the data presented here makes them a low risk for successful implementation in viral vector manufacturing. Future work could include additional development of lentivirus clarification to further improve yield. Work could include fractionating the filtrate from various filter chemistries and pore sizes to investigate how yield loss may be split between adsorption and size exclusion. Previously work [19]Raghavan B, Collins M, Walls S, Lambropoulos A, Bergheim-Pietza S. Development of gene therapy purification processes. Cell Gene Ther. Insights. 2019; 5(9), 1311–22. has demonstrated similar clarification development of AAV from suspension cultures, but clarification from suspension lentivirus cultures remains a target for future work. Data demonstrating full scalability from development scale to manufacturing scale would also be of value. In this work we observed some trending between feedstock turbidity and filter capacity, but there were some notable deviations suggesting that additional feedstock characterization would be needed to predict impact on filtration performance. Particle concentration and size distribution in crude harvests and their relation to filter performance could be an interesting follow up. Finally, we highlight the need for new technologies. This may include new filter media to improve viral yields. Furthermore, as gene therapy manufacturing has limited options for adventitious virus and endotoxin clearance there is a strong desire for closed, aseptic processing, highlighting the need for depth filter options that fit these requirements.
iCellis, Fluorodyne, PreFlow, Seitz, Supor, Stax and Supracap are trademarks of Pall Corporation PEIpro is a trademark of Polyplus Corning is a trademark of Corning Inc., CellSTACK is a trademark of Corning Life Sciences Hach is a trademark of Hach and Pendotech is a trademark of Pendotech |
References
1.Barlow JF, Tredenick-Fricke TL, Glover C, Hitchcock T, King D, Krishnan M, et al. Insights on Successful Gene Therapy Manufacturing and Commercialization. 2020. 1–44. https://go.pall.com/genetherapyebook.html?utm_source=cellculturedish&utm_medium=social&utm_campaign=20-10-121-GTEBO&utm_term=ebook&utm_content=cellculturedish Crossref
2.Forsber N, King D, Glover C, Madsen J, Hughes J V, Moore A, et al. Key Considerations in Gene Therapy Manufacturing for Commercialization. Cell Cult. Dish. 2018; Crossref
3.Samulski RJ, Muzyczka N. AAV-mediated gene therapy for research and therapeutic purposes. Annu Rev. Virol. 2014; 1(1), 427–51. Crossref
4.Merten O-W. AAV vector production: state of the art developments and remaining challenges. Cell Gene Ther. Insights. 2016; 2(5), 521–51. Crossref
5.Frecha C, Szecsi J, Cosset F-L, Verhoeyen E. Strategies for Targeting Lentiviral Vectors. Curr. Gene Ther. 2008; 8(6): 449–60. Crossref
6.Rabinowitz J, Chan YK, Samulski RJ. Adeno-associated Virus (AAV) versus immune response. Viruses 2019; 11(2), 1–11. Crossref
7.Rayaprolu V, Kruse S, Kant R et al. Comparative Analysis of Adeno-Associated Virus Capsid Stability and Dynamics. J. Virol. 2013; 87(24), 13150–60. Crossref
8.Bennett A, Patel S, Mietzsch M et al. Thermal Stability as a Determinant of AAV Serotype Identity. Mol. Ther. - Methods Clin. Dev. 2017; 6, 171–82. Crossref
9.de las Mercedes Segura M, Kamen A, Garnier A. Downstream processing of oncoretroviral and lentiviral gene therapy vectors. Biotechnol Adv. 2006; 24(3), 321–37. Crossref
10.Besnard L, Fabre V, Fettig M et al. Clarification of vaccines : An overview of filter based technology trends and best practices. Biotechnol. Adv. 2016; 34, 1–13. Crossref
11.Zydney AL. New developments in membranes for bioprocessing – A review. J. Memb. Sci. 2020; 118804. Crossref
12.Liu HF, Ma J, Winter C, Bayer R. Recovery and purification process development for monoclonal antibody production. MAbs. 2010; 2(5), 480–99. Crossref
13.Kumru OS, Wang Y, Gombotz CWR et al. Physical Characterization and Stabilization of a Lentiviral Vector Against Adsorption and Freeze-Thaw. J. Pharm. Sci. 2018; 107(11), 2764–74. Crossref
14.Potter M, Lins B, Mietzsch M et al. A simplified purification protocol for recombinant adeno-associated virus vectors. Mol. Ther. - Methods Clin. Dev. 2014; 1, 14034. Crossref
15.Nam H-J, Lane MD, Padron E et al. Structure of Adeno-Associated Virus Serotype 8, a Gene Therapy Vector. J. Virol. 2007; 81(22): 12260–71. Crossref
16.Khanal O, Singh N, Traylor SJ et al. Contributions of depth filter components to protein adsorption in bioprocessing. Biotechnol. Bioeng. 2018; 115(8), 1938–48. Crossref
17.Nejatishahidein N, Borujeni EE, Roush DJ, Zydney AL. Effectiveness of Host Cell Protein Removal using Depth Filtration with a Filter Containing Diatomaceous Earth. Biotechnol. Prog. 2020; 36(6), e3028. Crossref
18.Labisch JJ, Bollmann F, Wolff MW, Pflanz K. A new simplified clarification approach for lentiviral vectors using diatomaceous earth improves throughput and safe handling. J. Biotechnol. 2021; 326, 11–20. Crossref
19.Raghavan B, Collins M, Walls S, Lambropoulos A, Bergheim-Pietza S. Development of gene therapy purification processes. Cell Gene Ther. Insights. 2019; 5(9), 1311–22. Crossref
20.Moreira AS, Faria TQ, Oliveira JG, Kavara A, Schofield M, Sanderson T, et al. Enhancing the purification of Lentiviral vectors for clinical applications. Sep. Purif. Technol. 2021; 274, 118598. Crossref
21.Lutz H. Rationally defined safety factors for filter sizing. J. Memb Sci. 2009;341(1–2), 268–78. Crossref
Affiliations
Rajeshwar Chinnawar, Sr R&D Engineer, Pall Corporation
Nicholas Marchand, R&D Manager, Pall Corporation
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: The authors disclose that Pall Corporation owns patents relevant to the Biotech
Industry. The authors have no other conflicts of interest.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2022 Pall Corporation. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Mar 7 2022; Revised manuscript received: Apr 12 2022; Publication date: May 5 2022.

