Possibilities for continuous closed-system processing of cell therapies
Cell & Gene Therapy Insights 2022; 8(7), 799–807
DOI: 10.18609/cgti.2022.121
Cell and gene therapies have the potential to facilitate disease-modifying treatment of both rare and chronic conditions. Whilst approved treatments are now available, several challenges remain before these therapeutic modalities become first in line therapies. The supply and qualification of starting materials such as peripheral blood and bone marrow is one dilemma that must be overcome. For allogeneic programs, starting materials generally have a defined limit of productivity and carry requirements of substantial risk-mitigation testing. Autologous manufacturing processes, with lower attendant safety risks, are highly variable and may be compromised based on individual patient treatment programs or the target disease itself. An ideal starting material has a high capacity for expansion, providing consistent manufacturing, reduced qualification burden, and a high output of individual doses. However, even if a developer is working with an ideal starting material, manufacturing processes may limit the ability to capitalize on beneficial characteristics. One possible solution to improving manufacturing capacity is incorporation of continuous batch manufacturing.
Cell and cell-based gene therapies have the potential to facilitate disease-modifying treatment of numerous serious conditions. Whilst approved treatments are now available, several challenges remain before these therapeutic modalities become first-in-line therapies. Robust manufacturing at scale to treat the relevant patient populations remains one of the most vexing challenges [1]Lee B, Jung S, Hashimura Y et al. Cell Culture Process Scale-Up Challenges for Commercial-Scale Manufacturing of Allogeneic Pluripotent Stem Cell Products. Bioengineering 2022; 9(3), 92.. One possible solution to improving manufacturing capacity is incorporation of continuous, closed-system manufacturing processes. Whereas continuous manufacturing processes are widely used in numerous industries, only now are cell and cell-based gene therapy developers beginning to explore these concepts. In this white paper, we outline a concept, utilizing existing commercial tools, that could potentially accomplish such a manufacturing process [2]Khanal O, Lenhoff AM. Developments and opportunities in continuous biopharmaceutical manufacturing. MAbs 2021; 13(1), 1903664..
Modern development pathways benefit from a Quality by Design approach (QbD) at an early stage of development. Often however, the reality is that many groups are faced by significant pressures from multiple stakeholders to attain clinical and commercial development milestones. Thus, the identification and confirmation of Critical Quality Attributes (CQAs), and the Critical Process Parameters (CPPs) that allow these to be met, may be sub-optimal at early and mid-stage clinical phases [3]Bure K, Lipsitz Y, Lowdell M et al. Quality By Design for Advanced Therapies: An Informed Route to Enhanced Late-Stage Clinical Success and Empowered Process Flexibility. BioProcess Int. 2020.. Pressures for commercial success can lead to the direct transfer of research products and processes into early-stage clinical development platforms, with a retrospective, rather than proactive approach to defining CPP. Process or scale changes can lead to significant differences in analyzed parameters. Where those parameters are based on experience rather than empirical evidence of the boundary conditions, interpreting the implication can be daunting. To date, this has resulted in serious challenges in process improvement, modification, or even scale changes [2]Khanal O, Lenhoff AM. Developments and opportunities in continuous biopharmaceutical manufacturing. MAbs 2021; 13(1), 1903664..
Continuous manufacturing processes provide a number of key benefits, if they can be properly implemented. Traditional batch manufacturing requires an entire process to be complete before a product can be released and a new batch can be started. As it relates to biopharmaceutical manufacturing, this can mean very long manufacturing cycles before a new batch is initiated. Furthermore, changeover procedures mean that expensive manufacturing infrastructure is not utilized every time a batch finishes until it can be re-set. With current manufacturing systems and the anticipated manufacturing scale for early allogeneic cell-based therapies, this could severely limit the manufacturing capacity without massive build out of infrastructure [4]Kim M, Carrick K, Park M. Advanced Therapies: Industry & Regulatory Members Share Perspectives At NIFDS-USP Joint Workshop. Cell & Gene 2022.. By contrast, establishing a continuous manufacturing process would mean release of products earlier, and fewer changeover cycles. One intriguing possibility of continuous process implementation is the use of smaller scale systems to achieve the same manufacturing capacity of larger systems over a given period. In this way, a developer might be able to use the same manufacturing systems applied during clinical development as they use at commercial scale; higher annual throughput a function of longer cycles rather than larger systems.
As part of a concept demonstration, Sexton Biotechnologies (part of BioLife Solutions) worked alongside PBS Bioreactors, Pluristyx, and Luna Therapeutics, to present how a closed-system, continuous manufacturing process might be established. In this example, we establish a small-scale process appropriate for production of MSC or iPSC seed cells.
Materials & methods
Here we describe a potential workflow that outlines a continuous batch manufacturing cycle. If optimized, this process could reduce time to cell number targets, overall media requirements, and, by reducing the downtime and change-over burden, may increase manufacturing efficiency for a given manufacturing footprint. As with any manufacturing system, the ability for a process flow to accomplish a set of manufacturing parameters must be established on a per-product basis. Critical elements of process development such as determination of exhaustion limits, phenotypes based on changes in cell density, potential for cellular aggregation and the impact thereof, and many other aspects a developer would need to consider. These aspects were beyond the scope of the current project.
The Signata™ CT-5 (Sexton Biotechnologies) fluid management system allows weldable fluid line connections to close out many manual fluid management tasks. At the most basic level, it provides reproducible fluid movement with electronic records for traceability. However, it also functions as an independent unit operation platform for formulation and fill, closing processes such as media formulation, biopreservation media addition, and fill into bags or vials. The system is designed to embed flexible automation across cell therapy processes and allow integration of other standalone technologies within a single manufacturing platform.
PBS Bioreactors represent single use culture systems which can be scaled from 0.1 L to 80 L, with the vertical wheel technology enabling homogeneous particle suspension and low sheer stress. The integration of system design and function from very small scale to manufacturing scale limits challenges of platform migration throughout development.
Thermally induced phase separation (TIPS) microcarriers were originally developed at University College London and are now produced by Luna Therapeutics. Their material design incorporates hydrolytic resorption meaning cell detachment and particle separation may not be required (dependent on the requirements of any further downstream use). This offers the potential that they could be an ideal manufacturing and, potentially, delivery tool for cell-based therapies.
Pluristyx provides human iPSC for research and development applications, as well as offering custom manufacturing options for GMP cell banks for use in clinical manufacturing processes. The off-the-shelf nature of the cells offers developers a well characterized starting material for process modeling or development.
The project was divided across two workflows:
- The primary goal was to establish the ability of the PBS bioreactor to:
- Propagate adipose-derived MSC (AD-MSC) adherence and expansion on TIPS microcarriers (Figure 1
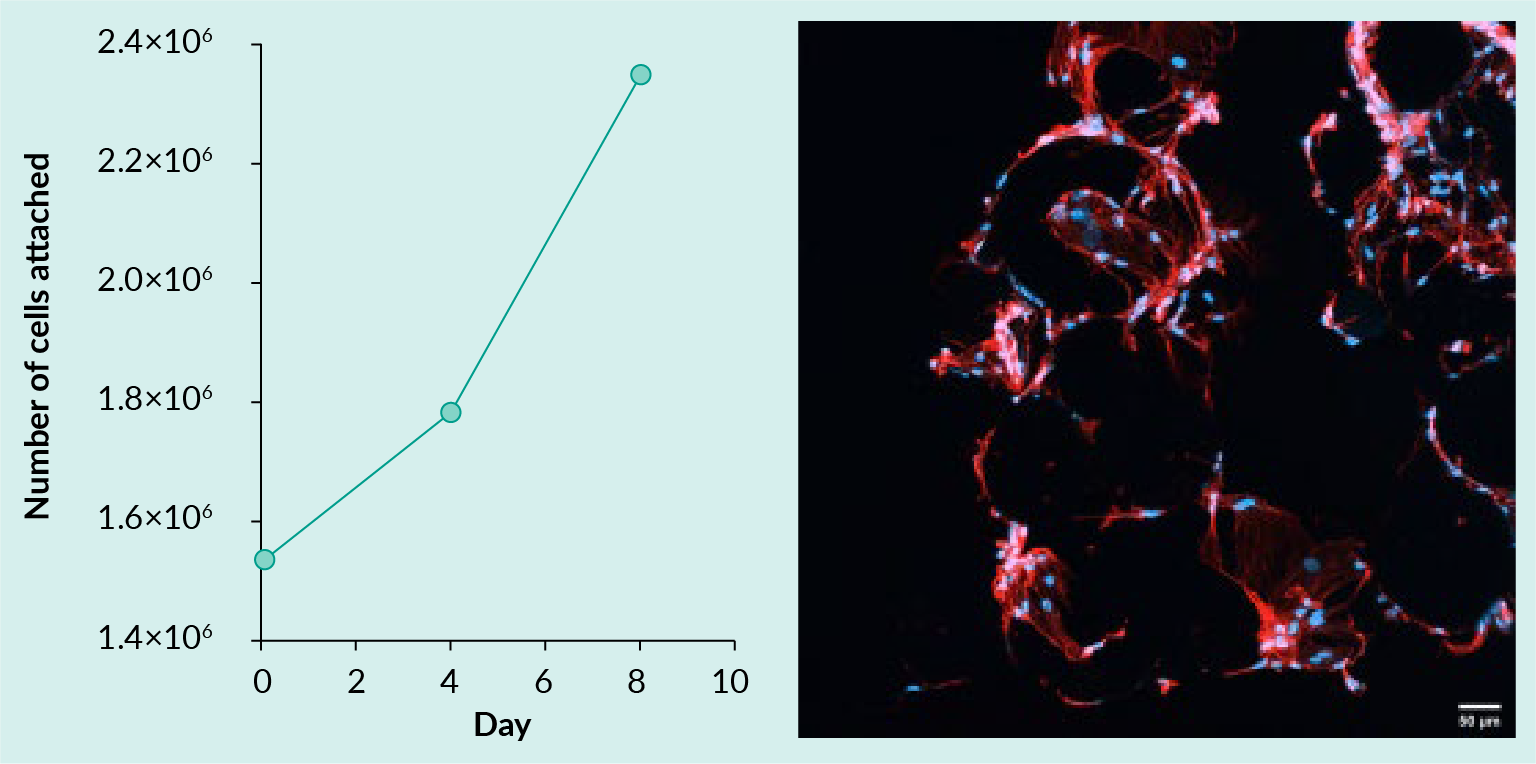 Adipose-derived MSC attached to TIPS microcarriers pre-treated with nLiven human platelet lysate (Sexton) and incubated in a PBS 0.1 MAGInitial seeding density 1.5 x 106 cells and 50 mg TIPS microcarriers. Cells imaged with confocal microscopy after staining nuclei with Hoechst 33342 (blue) and cytoskeleton with phalloidin (red). (Image courtesy of Haowei Wang).).
Adipose-derived MSC attached to TIPS microcarriers pre-treated with nLiven human platelet lysate (Sexton) and incubated in a PBS 0.1 MAGInitial seeding density 1.5 x 106 cells and 50 mg TIPS microcarriers. Cells imaged with confocal microscopy after staining nuclei with Hoechst 33342 (blue) and cytoskeleton with phalloidin (red). (Image courtesy of Haowei Wang).). - Propagate iPSCs in suspension culture (Figure 2
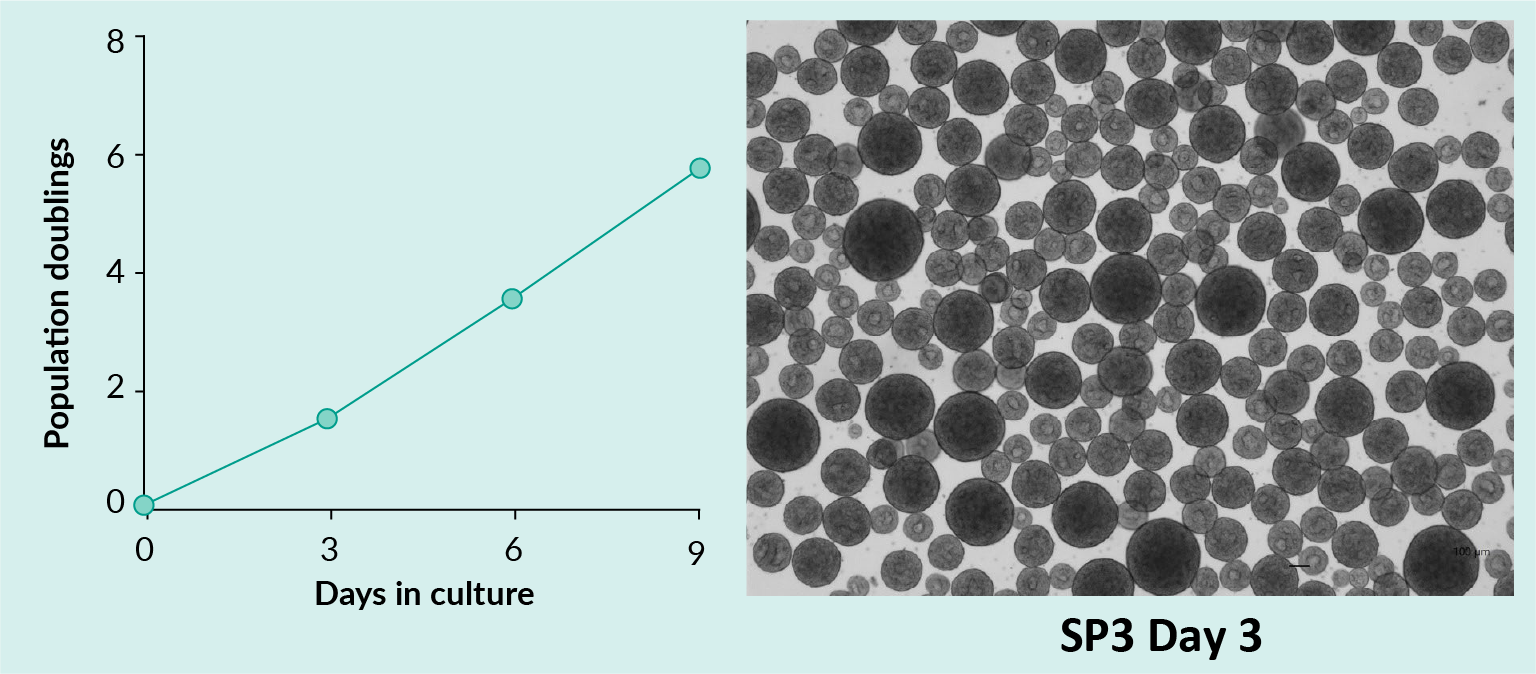 Expansion of induced Pluripotent Stem Cells in suspensionA vial of Pluristyx’s Ready-to-Differentiate® iPSCs were thawed directly into a PBS 0.1 MAG and cultured over 3 suspension passages. Population doubling was calculated (left panel) and iPSCs were imaged at suspension passage 3 (right panel) to demonstrate the morphology of iPSC clusters. Data and images courtesy of Dr Raluca Marcu.).
Expansion of induced Pluripotent Stem Cells in suspensionA vial of Pluristyx’s Ready-to-Differentiate® iPSCs were thawed directly into a PBS 0.1 MAG and cultured over 3 suspension passages. Population doubling was calculated (left panel) and iPSCs were imaged at suspension passage 3 (right panel) to demonstrate the morphology of iPSC clusters. Data and images courtesy of Dr Raluca Marcu.). - Once expansion was demonstrated, we sought to conceptualize how these commercially available tools could be adopted for a theoretical closed-system continuous process.
Outcomes
PBS Bioreactor enables adipose-derived MSC adherence & expansion on TIPS
A sterile, closed PVC tube was added to the access line of a PBS (Cartridge) in a biosafety cabinet to ensure sterile connection. Downstream additions or removal from the PBS system were made by sterile welding lines from the Signata CT-5 transfer sets or output sets. Samples were removed for analysis at D4 and D8. Figure 1 shows the expansion of AD-MSCs under these conditions as well as micrographs demonstration adherence to the TIPS microparticles.
PBS Bioreactor supports expansion & maintains pluripotency of iPSCs
For the iPSC expansion, the PBS system was loaded with media and inoculated with cells. Samples were removed at D3, D6, and D9. The photomicrograph demonstrates iPSC clusters.
Continuous closed-system bioprocessing using the Signata CT-5
The ability to perform manufacturing in a closed system with minimal labor input can allow for an economical process that reproducibly generates products meeting quality expectations. In this example, the Signata CT-5 was used to implement closed system bioprocessing of both the MSC and iPSC processes described above. Several preparation steps are needed to complete this workflow. Firstly, all reagents needed to be packaged in containers suitable for sterile welding. Suppliers are moving toward appropriate packaging, however, in some cases accessing materials from vials or bottles is still needed. For bottles, caps with weldable tubing are available. For vials, transfer into a closed system may need to be carried out in a Biological Safety Cabinet (BSC). New packaging options such as vials with weldable connections are improving in this part of the process, but availability is limited at this point. Second, in some cases, unit operation tubing sets may not be compatible with the downstream unit operation. In the case of interacting with the Signata CT-5, Sexton has used weldable lines with standard Luer connections. Tube size stepdown can be accomplished with Luer connected fittings if needed. Once these steps are completed, all processes can be accomplished with closed sterile welding.
The conceptual continuous manufacturing workflow is described in Figure 3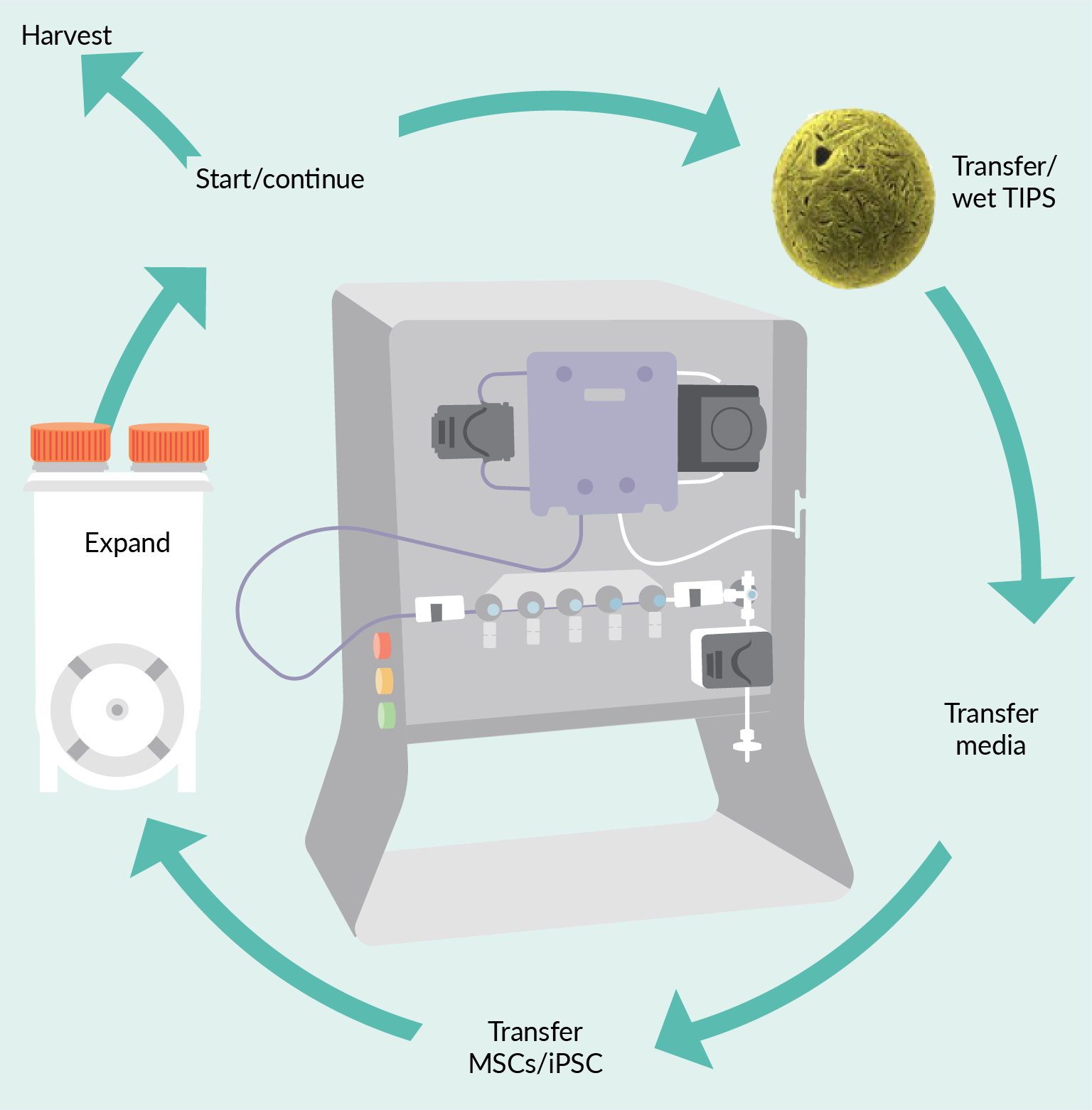 Concept for continuous bioprocessing of cell therapy.. In the case of manufacturing adherent cell lines such as MSCs, the microparticles must be prepared as per user instructions. Here, we used a vented vial adapter (connected in a BSC) to access the lyophilized TIPS particles. The wetting media (in this case nLiven™ hPL, Sexton Biotechnologies) was then added to the TIPS container using the CT-5. For non-adherent cell lines, this step would not be required.
Concept for continuous bioprocessing of cell therapy.. In the case of manufacturing adherent cell lines such as MSCs, the microparticles must be prepared as per user instructions. Here, we used a vented vial adapter (connected in a BSC) to access the lyophilized TIPS particles. The wetting media (in this case nLiven™ hPL, Sexton Biotechnologies) was then added to the TIPS container using the CT-5. For non-adherent cell lines, this step would not be required.
Once fully wetted, the particles are transferred to the PBS bioreactor, without the need to disconnect or open the process. Because the CT-5 has multiple available fluid lines, several processes can be completed without the need to re-weld onto the same line. In this case, different source positions were used for the TIPS particles, expansion media, and seed cells. Once the PBS bioreactor was filled, the fill line can be flushed. After preparation, the weldable fill line can be sealed and separated if needed (i.e., transfer into an incubator) or left connected if environmental conditions are supportive. The CT-5 can be used to draw QC samples during incubation if needed using the same weldable fill line.
At the established times or when samples reach specified criteria, cells are withdrawn. Because the system is established with weldable connections, after withdrawal of a portion of the expanded cells, additional media with or without wetted beads can easily be introduced to the reaction chamber. In this way, a single batch of cells from a working cell bank can be used continuously until the cells reach any pre-determined end-of-life, such as phenotype changes, exhaustion, etc. The process steps are described in the section below and illustrated in Figure 4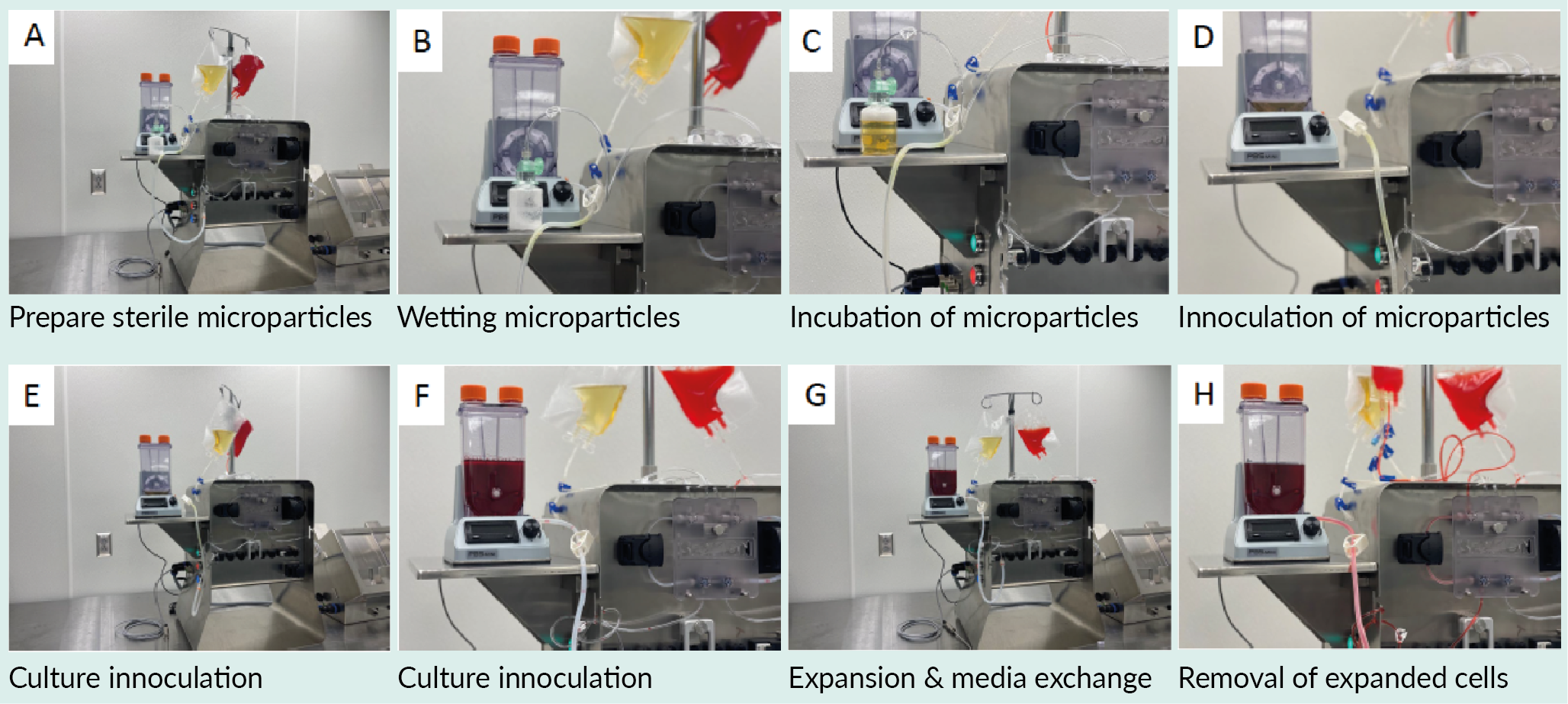 Set up of continuous processing concept..
Set up of continuous processing concept..
Process steps
Pre-condition TIPS microcarriers with platelet lysate
TIPS microcarriers were incubated in 100% nLiven platelet lysate until the particles settle to the bottom of the vial. Using a vented vial adapter connected to the output line on the Signata, platelet lysate is transferred in a functionally closed manner to the microparticle storage vial. Wetted microparticles are then transferred to a PBS Bioreactor connected to an output line on the Signata CT-5 DIY Output set. Alternatively, multiple microcarrier aliquots can be prepared from an initial conditioned suspension by connecting the source bottles to the Signata CT-5 CellSeal vial output set.
Load expansion vessel
Expansion media and seed cells prepared in source bags are welded onto the Signata CT-5 system as needed with the PBS Bioreactor connected as an output location.
Expansion
Culture expansion is carried out as per product specific optimized parameters. User guides and support for the PBS Bioreactors can be found at https://www.pbsbiotech.com/. Expand cells to desired density. Samples may be drawn using the connection to the Signata as needed.
Collection & re-seeding with microparticles
When cells reach desired density, a fraction of the bioreactor liquid may be drawn off and transferred to the desired wash/concentration system for further downstream processing (not shown). Formulation and fill can be completed using the Signata Formulation and Fill process into either cryobags or CellSeal® cryovials (Sexton Biotechnologies) for small volumes (up to 5 ml). To continue manufacturing, additional wetted particles and fresh media can be added to the Bioreactor. Expansion of cells can continue in this manner to limits defined by the user’s cell performance.
Continuous closed-system bioprocessing: impact on the field
In this short paper, we brought together technology from several tools providers to develop a process which was closed, definable and scalable. Through welding to the PBS bioreactor lines as well as all of the source and output lines, the Signata CT-5 integrates disparate tools and reagents. This enables closed introduction of source materials, media sampling and replenishment, collection, formulation, and fill. While the concept as shown does not include a wash/concentration step, compatibility with other systems is an inherent aspect of the built-in flexibility of the Signata CT-5. The range of PBS bioreactor vessels allows for small scale experiments during process development to be readily scaled once CPPs have been ascertained.
This presents several manufacturing possibilities.
- The ability to continually propagate cells in a closed and reproducible manner within appropriately sized PBS culture systems. These can be harvested into CellSeal vials or bags using the Signata CT-5 and cryopreserved.
- Continuous manufacturing enables higher output of cell product in smaller reactor vessels. Smaller, continuous outputs that can be formulated into final products. This suggests significantly faster production of final product, as well as reduced downtime due to changeover processes. In addition, the anticipated smaller scale of a reactor is a valuable risk management strategy: Implementation of multiple smaller scale reactors reduces the overall loss potential should a contamination or other manufacturing error occur. A scaled out continuous throughput system may have a higher initial financial investment (or, smaller sequential investments) but increases in efficiency can ultimately balance this out [5].
- As successful cell-based products expand into new indications, microcarrier expansion systems are understood to be one method of greatly expanding manufacturing capacity [6]. Should microcarriers be compatible with final formulation (i.e., without the requirement to separate from the cell-based product), downstream formulation and delivery may also become more efficient.
- This proposed strategy for continuous bioprocessing also maintains the flexibility to be scaled up or out. Larger PBS bioreactors with related volumes of iPSCs/TIPS microcarriers can allow up to 80 L scale up, whilst the ability to weld on and off multiple PBS Mini units can facilitate scale out, with both approaches utilizing the Signata CT-5 to maintain closed-system processing.
Further work is required to optimize processing parameters, but by harnessing the expertise of several technology providers, we have demonstrated how continuous closed-system processing can address several unmet needs within the cell therapy space.
Indeed, implementation of large-scale batch manufacturing systems early in development still represents a significant risk [7]Heathman TRJ, Stolzing A, Fabian C et al. Scalability and process transfer of MSC production from monolayer microcarrier culture using hPL. Cytotherapy 2016; 18(4), 523–535.. If scale up cannot be optimized, the infrastructure will be redundant, and as such smaller, scale-out models will be required. On the other hand, continuous, closed-system manufacturing has the potential to allow implementation of standardized processes early in development, with higher throughput in the same systems through longer batch cycles. As demonstrated, such a process could involve relatively small reaction vessels incorporated much earlier in development to limit the risk of scale-up failures. Incorporation of these systems minimizes human intervention, simplifies batch records, and increases the probability of success when higher throughput is needed.
References
1. Lee B, Jung S, Hashimura Y et al. Cell Culture Process Scale-Up Challenges for Commercial-Scale Manufacturing of Allogeneic Pluripotent Stem Cell Products. Bioengineering 2022; 9(3), 92. Crossref
2. Khanal O, Lenhoff AM. Developments and opportunities in continuous biopharmaceutical manufacturing. MAbs 2021; 13(1), 1903664. Crossref
3. Bure K, Lipsitz Y, Lowdell M et al. Quality By Design for Advanced Therapies: An Informed Route to Enhanced Late-Stage Clinical Success and Empowered Process Flexibility. BioProcess Int. 2020. Crossref
4. Kim M, Carrick K, Park M. Advanced Therapies: Industry & Regulatory Members Share Perspectives At NIFDS-USP Joint Workshop. Cell & Gene 2022. Crossref
5. Joshi AK, Gupta SK. Single-Use Continuous Manufacturing and Process Intensification for Production of Affordable Biological Drugs. In: Process Control, Intensification, and Digitalisation in Continuous Biomanufacturing. Subramanian G (Ed.); 2022. Crossref
6. Allison G, Cain YT, Cooney C et al. Regulatory and Quality Considerations for Continuous Manufacturing. May 20–21, 2014 Continuous Manufacturing Symposium. J. Pharm. Sci. 2015; 104(3), 813–820. Crossref
7. Heathman TRJ, Stolzing A, Fabian C et al. Scalability and process transfer of MSC production from monolayer microcarrier culture using hPL. Cytotherapy 2016; 18(4), 523–535. Crossref
8. Challener C. Evaluating the Rewards vs. the Risks of Automation. BioPharm Int. 2018; 31(9). Crossref
Affiliations
Sean Werner
Chief Technology Officer, Cell
Processing, BioLife Solutions
Steven Thompson
Vice-President, Sales (Cell
Processing), Sexton Biotechnologies
Richard Day
Chief Scientific Officer and Founder, Luna Therapeutics
Brian Hawkins
Chief Technology Officer, Pluristyx
Joseph Petrosky
Global Vice President of Sales, PBS Biotech
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: Authors are employees of respective organizations and may receive stock compensation as standard employment agreement.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2022 Sexton Biotechnologies. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Apr 19 2022; Revised manuscript received: Jul 11 2022; Publication date: Aug 22 2022.

